Markers and methods for predicting primary drug resistance of immune checkpoint inhibitor therapy
A technology of immune checkpoints and inhibitors, applied in the biological field, can solve the problems of no unified conclusion on TMB threshold, lack of standard methods for TMB, and different products and algorithms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] Example 1. Characterization of Discovery Cohort dMMR / MSI-H Gastrointestinal Tumor Patients
[0121] Table 1. Basic characteristics of patients with dMMR / MSI-H gastrointestinal tumors in the discovery cohort
[0122]
[0123]
[0124] dMMR: mismatch repair deficiency; MSI-H: high microsatellite instability; GI: gastrointestinal; ICI: immune checkpoint inhibitor; ECOG PS: Eastern Cooperative Oncology Group performance status; IC: intestinal cancer; GC: gastric cancer; PD-1: programmed death 1; PD-L1: programmed death ligand 1; HER2: human epidermal growth factor receptor 2; CTLA4: cytotoxic T lymphocyte antigen-4; TPS: tumor proportion score; LS: Lin odd syndrome.
[0125] Most patients (62 / 65, 95.38%) had good physical condition (ECOG 0 or 1) and received at least one systemic therapy in the past (54 / 65, 83.08%). 1 patient was HER2 positive (1 / 53, 1.89%) and 8 patients (8 / 49, 16.33%) had PD-L1 TPS ≥ 1%. 14 cases (21.54%) were diagnosed with Lynch syndrome (LS). ...
Embodiment 2
[0126] Example 2. Screening of genes that can predict immunotherapy resistance in dMMR / MSI-H gastrointestinal cancer patients
[0127] Screening of primary drug resistance predictive genes by a three-step approach:
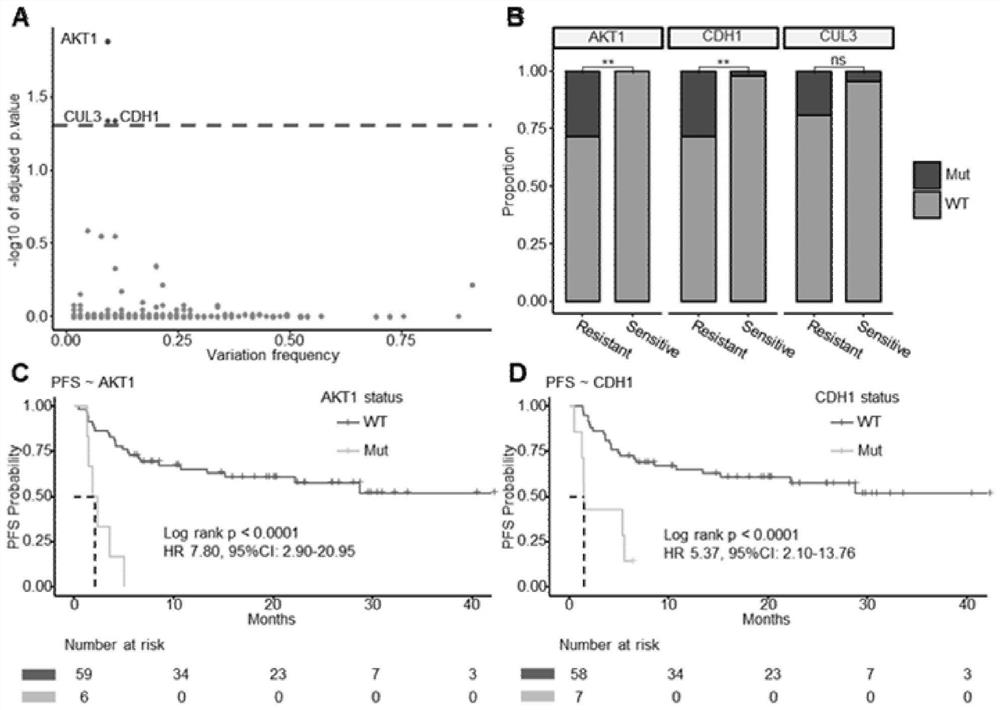
[0128] Step 1: Screening genomic mutations significantly associated with PFS by univariate Cox proportional hazards regression model (Pfigure 1 A).
[0129] Step 2: Compare the differences in the mutation frequencies of the three genes screened in the first step in the ICI-resistant group and the ICI-sensitive group, identify genes with significant mutation frequencies (Pfigure 1 B).
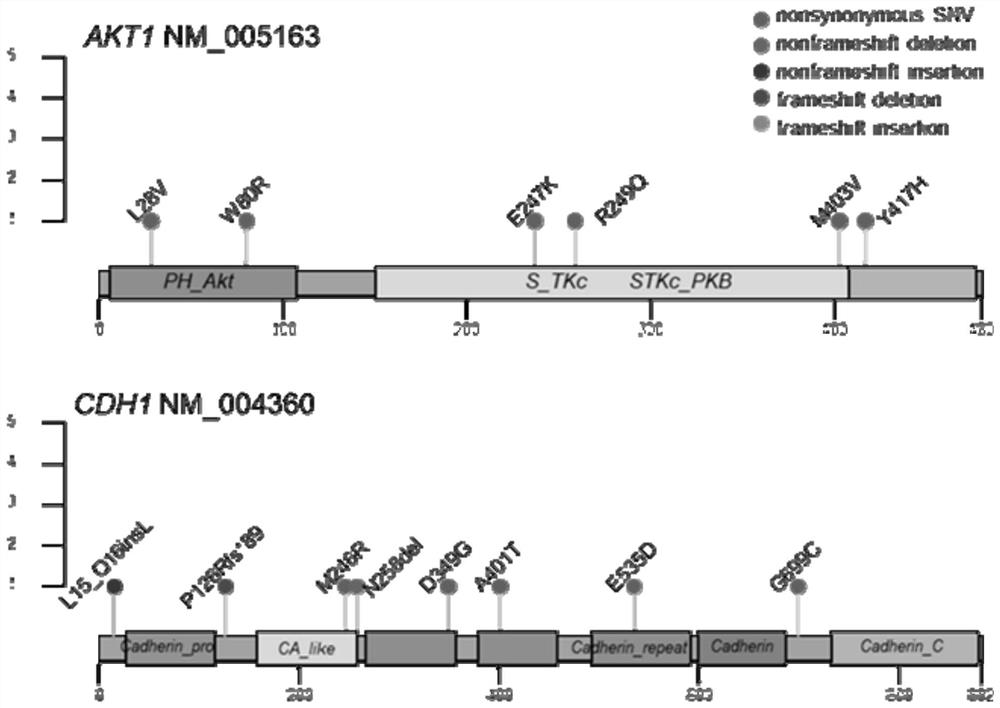
[0130] Step 3: Gene mutations need to meet the mutation frequency ≥5% criterion to ensure that the proportional difference between the two groups is not caused by randomly occurring mutations. Mutation frequencies of both AKT1 and CDH1 were ≥5%. Further analysis of the AKT1 and CDH1 gene mutation sites found that the gene mutation sites are scattered in the full length of AKT1 and...
Embodiment 3
[0132] Example 3. Construction of a combined AKT1 and CDH1 mutation model to predict immunotherapy resistance in dMMR / MSI-H gastrointestinal cancer patients
[0133] In the discovery cohort, there were 5 patients with AKT1-mut (mut:mutation), 6 patients with CDH1-mut, and 1 patient with co-mutation of AKT1 and CDH1.
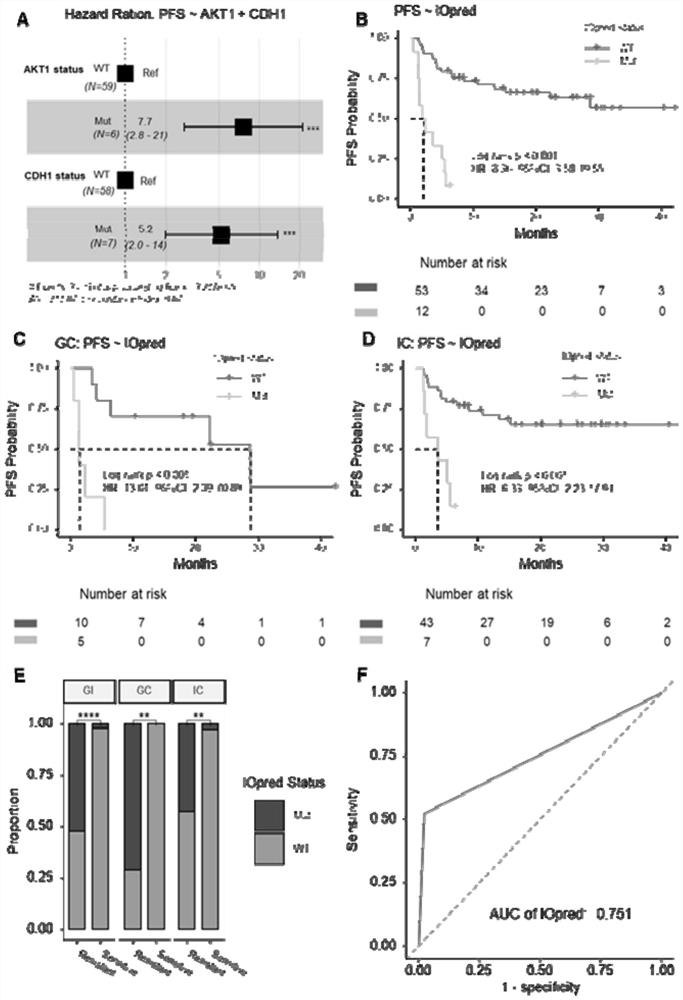
[0134] Given that the following two conditions were met: 1. Multivariate Cox regression analysis showed that AKT1 and CDH1 were independent predictors of PFS (P image 3 A); 2, Multivariate logistic regression analysis showed that AKT1 and CDH1 were independently associated with primary drug resistance (P image 3 B-D). To further evaluate the predictive value of Iopred for the efficacy of immunotherapy, we performed a univariate Cox regression analysis between Iopred and clinicopathological parameters, and the results showed that Iopred was the only predictor of PFS in patients with dMMR / MSI-H gastrointestinal tumors treated with ICI (Table 1). 2). IOpred mutatio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com