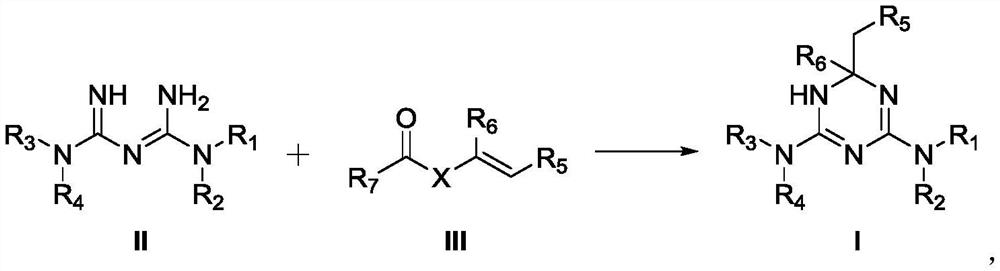
Preparation method of triazine derivative
A compound and alkyl technology, applied in the field of preparation of triazine derivatives, can solve the problems of reducing the probability of side effects and reducing the dosage of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
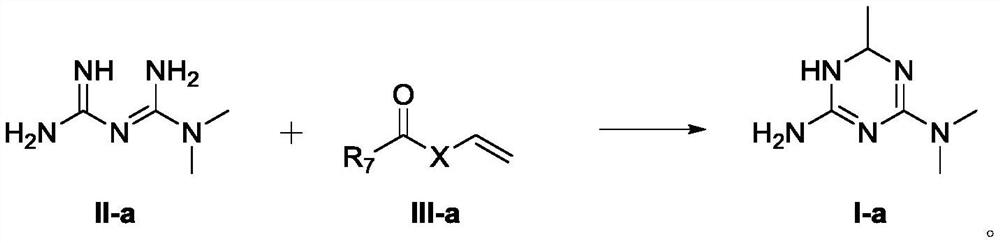
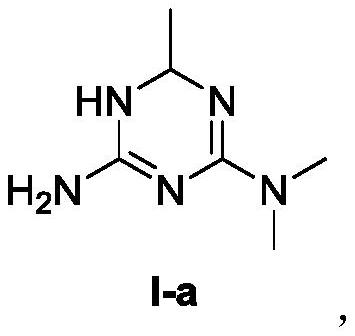
[0127] Example 1 - Preparation of 1,6-dihydro-2-amino-4-dimethylamino-6-methyl-1,3,5-triazine
[0128] Option A:
[0129]
[0130] Metformin hydrochloride (16.5 g, 0.1 mol) was added to a methanol / water mixed solvent, and an aqueous solution of potassium hydroxide (1 eq) and a methanol solution of vinyl acetate (1 eq) were added dropwise, and then reacted at room temperature. After the reaction, the reaction solution was concentrated under reduced pressure, then ethanol was added and heated to 60° C., filtered while hot, and the filtrate was concentrated to obtain the compound of formula I-a (14.7 g, yield 95%).
[0131] HRMS(ESI)Calcd.for C6H14N5 + [M+H] + 156.1249; Found: 156.1256.
[0132] 1 H NMR (400MHz, D 2 O): 4.93-4.88(m, 1H), 3.07(s, 6H), 1.45-1.44(d, 6Hz, 3H).
[0133] Option B:
[0134]
[0135] Metformin acetate (18.9 g, 0.1 mol) was added to methanol, and ammonia water (2 eq) and vinyl acetate (1.2 eq) were added dropwise, and then reacted at 60°C. A...
Embodiment 2
[0139] Example 2—Preparation and Resolution of 1,6-dihydro-2-amino-4-dimethylamino-6-methyl-1,3,5-triazine
[0140] (1) Metformin acetate (18.9 g, 0.1 mol) was added to methanol, and ammonia water (2 eq) and vinyl acetate (1.2 eq) were added dropwise, and then reacted at 60°C. After the reaction was completed, the reaction solution was concentrated under reduced pressure, the solution was concentrated to dryness, isobutanol was added and refluxed until fully dissolved, cooled to 65 ° C, acetone was added, the temperature was lowered to 0 ° C again, stirred for 1 hour, filtered, and the filter cake was washed twice with acetone , and dried to obtain the compound of formula I-a (17.6 g, yield 82%).
[0141] (2) Dissolve the racemic compound of formula I-a (8.43g, 39.2mmol) in methanol, add a methanol solution of di-O,O'-p-toluoyl-D-tartaric acid (10.65g, 27.6mmol), After stirring and crystallization for 2 hours, the filter cake was dried to obtain di-p-toluoyl-D-tartrate (10.3g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com