C3 symmetric bifunctional catalyst as well as preparation method and application thereof
A bifunctional catalyst, catalyst technology, applied in physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, chemical instruments and methods, etc. Complete recovery and other problems, to achieve the effect of mild and simple synthesis method, good catalytic activity and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
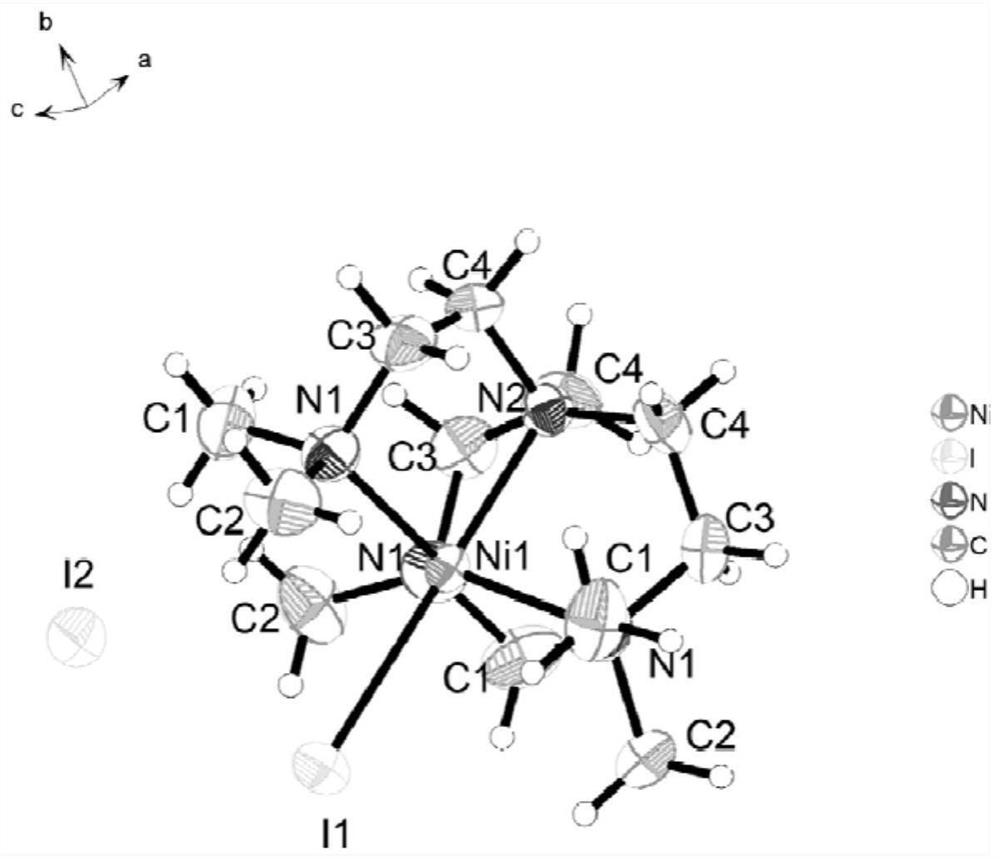
[0035] In this embodiment, the [Ni(Me 6 Tren)I]I, other catalysts in the present invention can be selected and catalysts [Ni(Me 6 Tren)I]I is prepared in the same synthetic manner, and the corresponding amount of synthetic raw materials should be used during preparation.
[0036] Catalyst [Ni(Me 6 The preparation method of Tren)I]I: first weigh 1.6mmol (0.5g) of NiI 2 In a 50 mL round-bottomed flask, dissolve it with 20 mL of ethanol, and then take 1.6 mmol (0.410 mL) of Me 6 Tren was added dropwise to the above solution. The reaction was stirred at 55°C for 48h. After the reaction was completed and cooled, the post-reaction solution was filtered. After filtration, the filtrate was concentrated to about 1 / 5, and 40 ml of methyl tert-butyl ether was added to precipitate the product. After the product was completely precipitated, the precipitate was washed 3-4 times with methyl tert-butyl ether. The catalyst [Ni(Me 6 Tren)I]I. The obtained catalyst [Ni(Me 6 The single ...
Embodiment 2
[0039] Catalyst [Co(Me 6 Tren) I] I preparation method: first weigh 1.6 mmol (0.5 g) of CoI 2 In a 50 mL round-bottomed flask, dissolve it with 20 mL of ethanol, and then take 1.6 mmol (0.410 mL) of Me 6 Tren was added dropwise to the above solution. The reaction was stirred at 55°C for 48h. After the reaction was completed and cooled, the post-reaction solution was filtered. After filtration, the filtrate was concentrated to about 1 / 5, and 40 ml of methyl tert-butyl ether was added to precipitate the product. After the product was completely precipitated, the precipitate was washed 3-4 times with methyl tert-butyl ether. The catalyst [Co(Me 6 Tren)I]I.
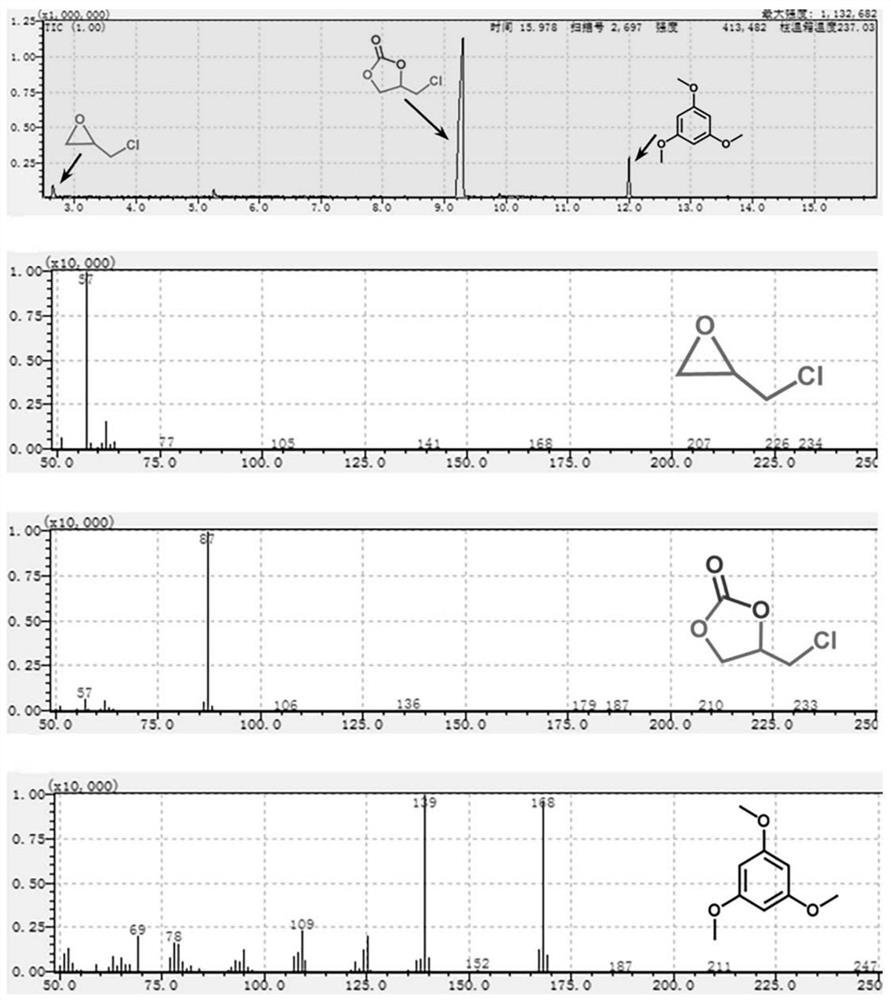
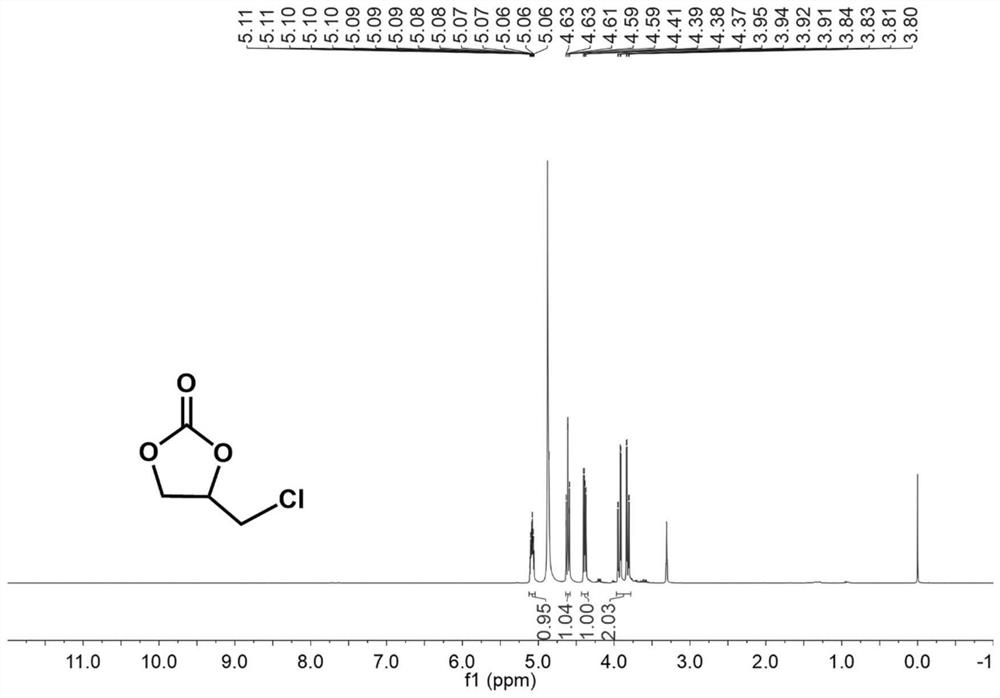
[0040] Into a 10 mL Schlenk reaction tube, 0.05 mol of catalyst [Co(Me 6 Tren)I]I and 5 mmol of epichlorohydrin, after replacing the air in the reaction tube with carbon dioxide gas three times, a carbon dioxide balloon was inserted. The reaction was stirred for 24 h in an oil bath at 60 °C. After the reaction was co...
Embodiment 3
[0042] Catalyst [Ni(Me 6 Preparation method of Tren)Br]Br: First weigh 1.6mmol (0.349g) of NiBr 2 In a 50 mL round-bottomed flask, dissolve it with 20 mL of ethanol, and then take 1.6 mmol (0.410 mL) of Me 6 Tren was added dropwise to the above solution. The reaction was stirred at 55°C for 48h. After the reaction was completed and cooled, the post-reaction solution was filtered. After filtration, the filtrate was concentrated to about 1 / 5, and 40 ml of methyl tert-butyl ether was added to precipitate the product. After the product was completely precipitated, the precipitate was washed 3-4 times with methyl tert-butyl ether. The catalyst [Ni(Me 6 Tren)Br]Br.
[0043] Into a 10 mL Schlenk reaction tube, 0.05 mol of catalyst [Ni(Me 6 Tren)Br]Br and 5 mmol of epichlorohydrin, after replacing the air in the reaction tube with carbon dioxide gas three times, a carbon dioxide balloon was inserted. The reaction was stirred for 24 h in an oil bath at 80 °C. After the reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com