Process and equipment for preparing ivabradine intermediate benzazepine skeleton
A technology of ivabradine and its preparation process, which is applied in the field of drug preparation, can solve the problems of unfriendly environment and harsh reaction conditions, and achieve the effects of reducing production cost, less pollution of three wastes, and high atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
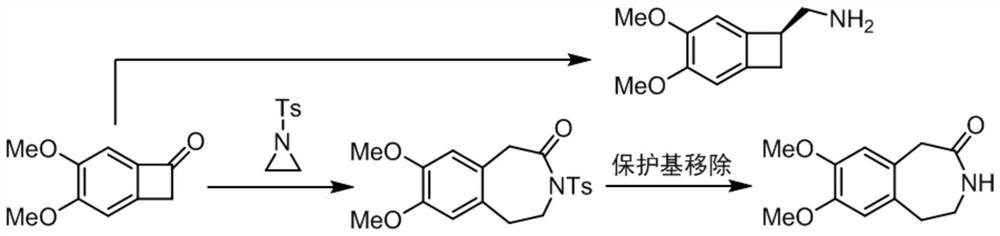
[0046] refer to Figure 1-Figure 10 As shown, a preparation process and equipment of an ivabradine intermediate benzazepine skeleton, specifically, the preparation process of the ivabradine intermediate benzazepine skeleton, comprising the following steps:
[0047]S1: Using benzocyclobutanone and N-heterocyclopropane as starting materials, under the synergistic catalysis of palladium / Lewis acid, a cross-dimerization ring expansion reaction occurs between benzocyclobutanone and N-heterocyclopropane to generate Ts (p-toluenesulfonyl) protected benzazepines, for subsequent use;
[0048] S2: Dissolve the Ts-protected benzoazepine compound in S1 in THF (tetrahydrofuran) solution, and cool the reaction solution to 0° C. for subsequent use;
[0049] S3: The reaction solution in S2 was added dropwise to the THF (tetrahydrofuran) solution of samarium diiodide in a state of stirring, until the titration was completed, and the reaction temperature was 0 °C and continued to stir for 1 h....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com