Synthesis method of GCGR/GLP-1R double-target agonist polypeptide
A technology of GLP-1R and synthesis method, which is applied in the field of synthesis of polypeptide compounds, can solve the problems of resin shrinkage, easy folding, prolonging reaction time, etc., and achieve the effects of reducing difficulty, improving purity and yield, and simplifying process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
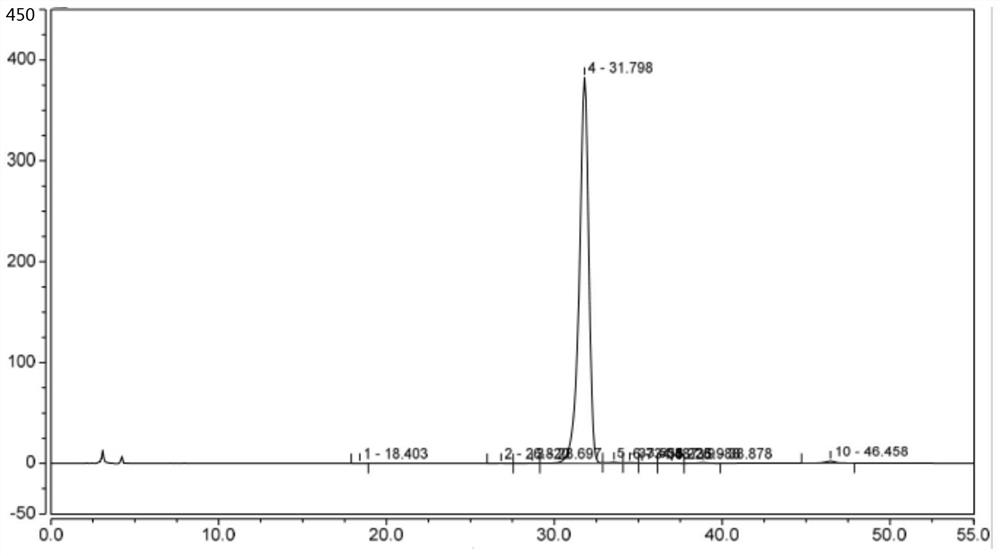
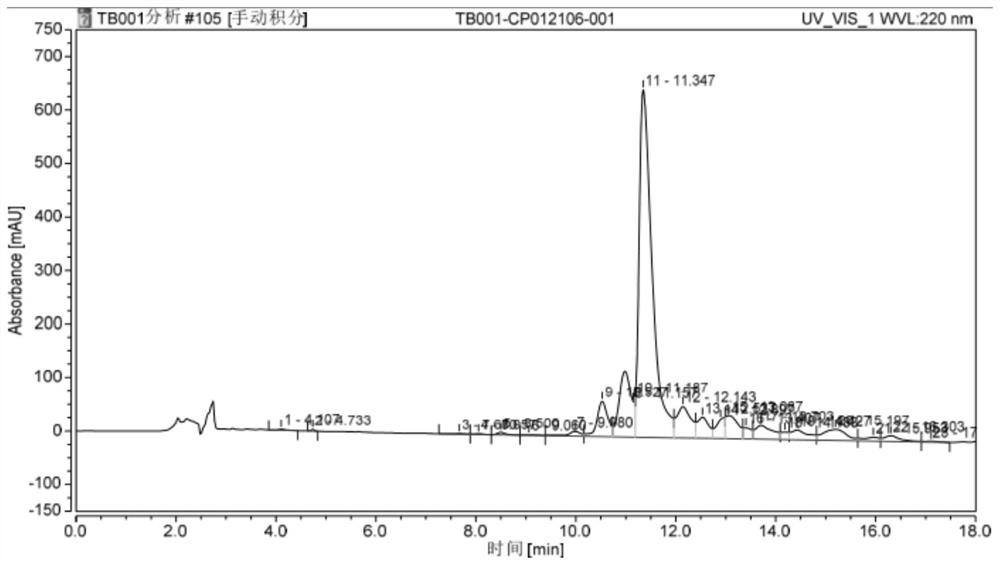
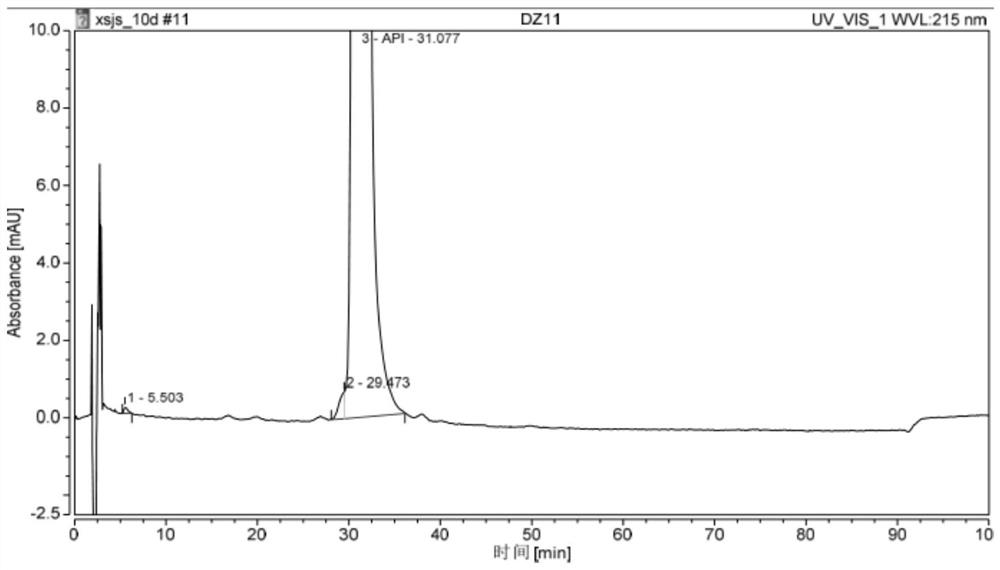
Image
Examples
Embodiment 1
[0080] The preparation of the Fmoc-Gly-CTC resin that the substitution degree of embodiment 1 is 0.8mmol / g
[0081] A. 10 g (13 mmol) of 2-CTC resin with a substitution degree of 1.3 mmol / g was added to the reaction kettle, 100 mL of DCM was added, after mixing for 2 min, the DCM was filtered off, then 100 mL of DCM was added, after 40 min of mixing, the DCM was filtered off, and finally added 100 mL of DCM, after mixing for 2 min, the DCM was filtered off, and the resin was used for later use.
[0082] B. Weigh 9.81g of Fmoc-Gly-OH and 5.35g of HOBT in a beaker, add 100mL of DMF and 5.46mL of DIEA, stir and activate the solution at 0-10°C for 5min, pour it into the CTC resin obtained in step A , and mixed for 4h at 20-25°C. After the reaction was completed, 100 mL of DMF was added, and the DMF was filtered off. 4 mL of methanol and 5.46 mL of DIEA were added and mixing continued for 1 h. After the reaction was completed, suction filtration, and the resin was washed with DM...
Embodiment 2
[0084] The preparation of the Fmoc-Gly-CTC resin that the substitution degree of embodiment 2 is 1.0mmol / g
[0085]A. 10g (16mmol) of 2-CTC resin with a substitution degree of 1.6mmol / g was added to the reactor, 100mL of DCM was added, after mixing for 2min, the DCM was filtered off, then 100mL of DCM was added, after 40min of mixing, the DCM was filtered off, and finally added 100 mL of DCM, after mixing for 2 min, the DCM was filtered off, and the resin was used for later use.
[0086] B. Weigh 9.81g of Fmoc-Gly-OH and 5.35g of HOBT in a beaker, add 100mL of DMF and 5.46mL of DIEA, stir and activate the solution at 0-10°C for 5min, pour it into the CTC resin obtained in step A , and mixed for 4h at 20-25°C. After the reaction was completed, 100 mL of DMF was added, and the DMF was filtered off. 4 mL of methanol and 5.46 mL of DIEA were added and mixing continued for 1 h. After the reaction was completed, suction filtration, and the resin was washed with DMF 5 times, 100 m...
Embodiment 3
[0088] Example 3 Preparation of S1-S4 peptide resin Boc-His(π-MBom)-D-Ser(tBu)-Gln(Trt)-Gly-CTC resin
[0089] A. All the Fmoc-Gly-CTC resin obtained in Example 1 was poured into the reaction kettle, swollen and mixed with 100 mL of DCM for 15 min and then drained. Add 100 mL of 20% piperidine / DMF solution (ie DBLK solution) by volume, mix at 20-30° C. for 5 min, and then drain. 100 mL of DMF was added, mixed for 5 min, and then drained. Add 100 mL of DBLK solution with a volume concentration of 100 mL, mix at 20-30 °C for 10 min, and then drain. 100 mL of DMF was added, mixed for 5 min, and then drained. Repeat washing with DMF 8 times, 100 mL each time, mixing for 5 min each time, and after the seventh washing, use pH test paper to detect the filtrate, and the result shows that the pH is 6.5-7.0 is qualified.
[0090] B. Weigh 12.20g of Fmoc-Gln(Trt)-OH, 3.03g of DIC and 2.7g of HOBT in a clean 1L beaker in turn, add 100mL of DMF / DCM solution with a volume ratio of 1:1, p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com