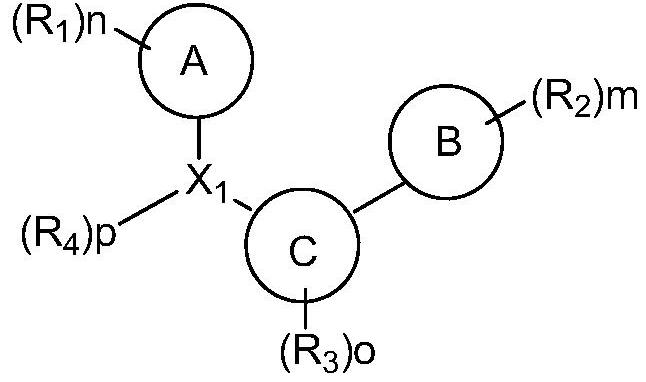
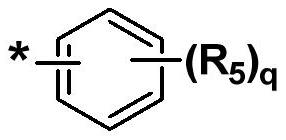
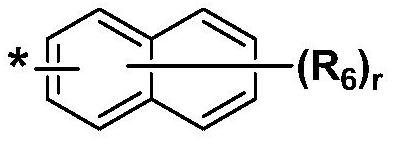
Organic compound and organic electroluminescent element comprising same
A compound and bonding technology, which is applied in the direction of silicon organic compounds, organic chemistry, electrical components, etc., can solve the problems of color purity and efficiency reduction of organic electrical components, charge imbalance of light-emitting layer, low HOMO value, etc., to improve luminous efficiency And life characteristics, reduce hole accumulation, reduce the effect of driving voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0158]
[0159] 1-1) Synthesis of compound 1-1
[0160]
[0161] 1-Bromoadamantane (215.1 g, 1000 mmol) was dissolved in 1,3-dichlorobenzene (220.5 g, 1500 mmol) under nitrogen flow. After that, aluminum chloride (4.0 g, 30 mmol) was added and stirred at a temperature of 60°C for 8 hours. After the reaction was completed, the organic layer was extracted with water and dichloromethane. with MgSO 4 The extracted solution was treated to remove moisture, dried and concentrated, and then purified by column chromatography. Then, the compound 1-1 of 182.8g (yield: 65%) was obtained by recrystallization from methanol.
[0162] 1-2) Synthesis of compound 1-2
[0163]
[0164] Under nitrogen flow, add N-phenyl-[1,1'-biphenyl]-4-amine, 21.0 g, 85.60 g to the round bottom flask mmol), compound 1-1 (26.48 g, 94.16 mmol), t-BuONa (16.45 g, 171.2 mmol), Pd 2 (dba) 3 (1.57 g, 1.71 mmol), Sphos (1.41 g, 3.42 mmol) and toluene (350 mL), and then stirred at 100°C to react. After ...
Synthetic example 2
[0168]
[0169]
[0170] Except using (9,9-dimethyl-9H-fluoren-2-yl)boronic acid ((9,9-dimethyl-9H-fluoren-2-yl)boronic acid, 5.83 g, 24.49 mmol) instead of [1,1 Except for '-biphenyl]-4-ylboronic acid, 7.54 g (yield: 57%) of compound 39 was prepared in the same manner as the synthesis method of compound 32.
Synthetic example 3
[0171]
[0172]
[0173] Except using (9-phenyl-9H-carbazol-3-yl)boronic acid ((9-phenyl-9H-carbazol-3-yl)boronicacid, 7.03 g, 24.49 mmol) instead of [1,1'-biphenyl] Except for -4-ylboronic acid, 7.82 g (yield: 55%) of compound 47 was prepared in the same manner as the synthesis method of compound 32 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com