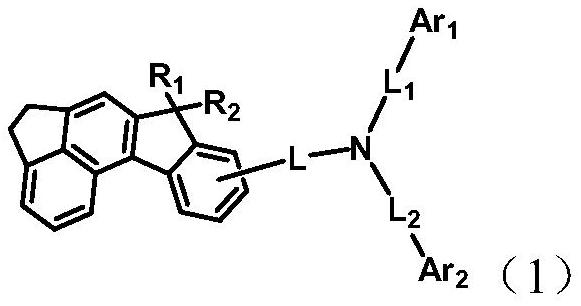
Arylamine compound containing indenofluorene group and organic electroluminescent device
An indenofluorene and compound technology, applied in the field of organic electroluminescent materials, can solve problems such as the influence of device life and the phase transition of hole transport materials, and achieve the effects of easy adjustment, increased glass transition temperature, and enhanced stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
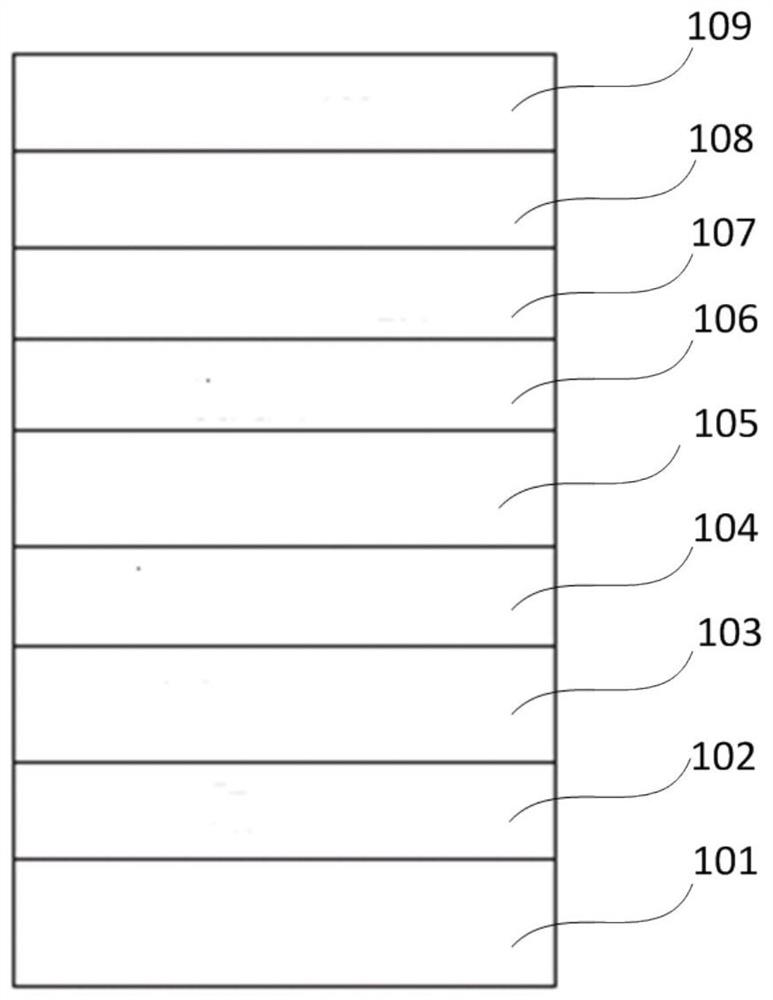
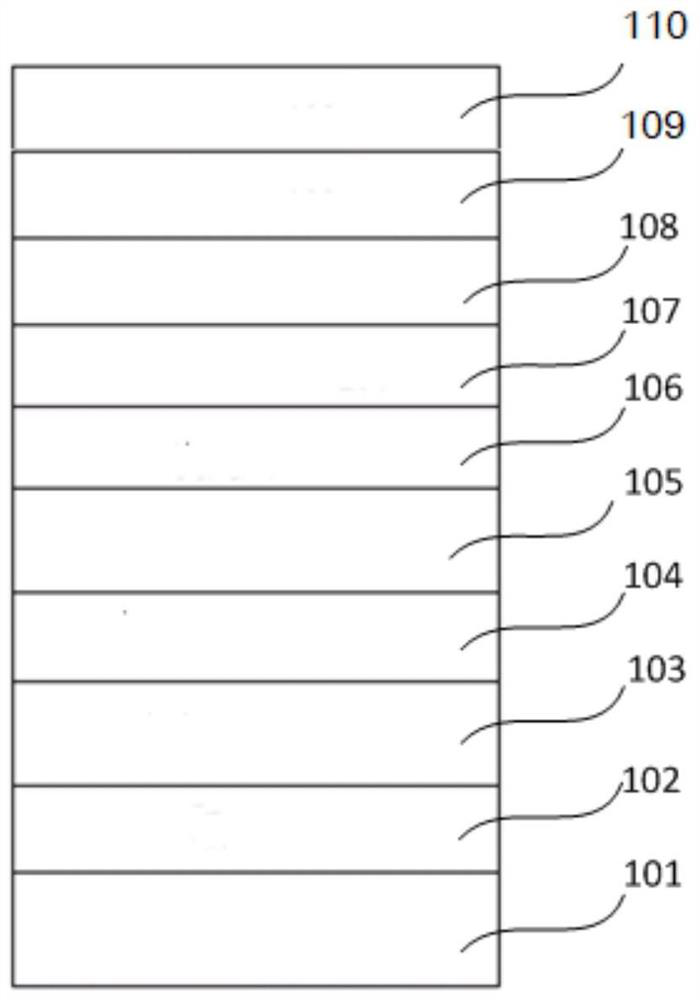
Image
Examples
Embodiment 1
[0034] Synthesis of Example 1 Compound HT-4
[0035]
[0036] (1) Synthesis of compound c2-4
[0037] Under a nitrogen atmosphere, acenaphthene-5-boronic acid (compound c1, 3.0g, 15.0mmol, 1eq), 2-bromo-4-chloro-1-iodobenzene (compound m2-4, 4.8g, 2-bromo-4-chloro-1-iodobenzene (compound m2-4, 4.8g, 15.0mmol, 1eq), and degassed toluene (100mL), after thorough mixing, sodium carbonate (2.4g, 22.5mmol, 1.5eq), tetrakis(triphenylphosphine)palladium (173.4mg, 0.15mmol, 1% eq), and degassed ethanol (40 mL) and deionized water (40 mL). The stirring was turned on, the above system was thoroughly mixed, and the reaction was refluxed for 6 hours under nitrogen atmosphere. After cooling to room temperature, toluene (100 mL) was poured into the reaction system. After standing for layers, extraction was performed with toluene (3×60 mL). The resulting organic phases were combined, dried over anhydrous magnesium sulfate, filtered, and the solvent was distilled off under reduced pressur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com