Benzene acrylamide compound as well as preparation method and application thereof
A technology of phenylacrylamide and acrylamide, which is applied in the field of preparation of phenylacrylamide compounds, can solve the problems that infectious diseases cannot be completely cured, affect the efficacy of antibacterial drugs, and irrational use of antibiotics, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
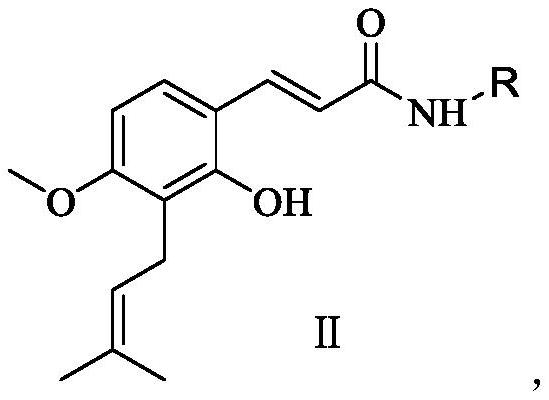
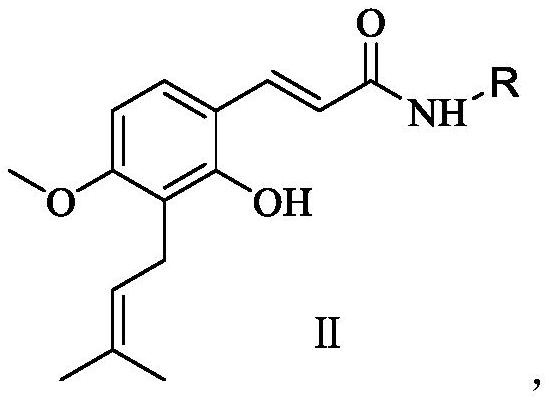
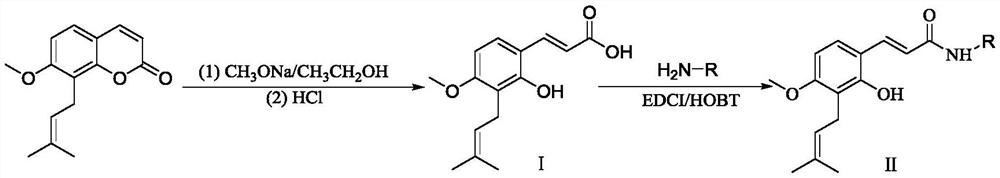
[0020] Preparation of compound II of the present invention:
[0021]
[0022] Wherein, EDCI is 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride, and HOBT is 1-hydroxybenzotriazole.
Embodiment 1
[0024] Preparation of compound II-1: Weigh 0.400g (1.64mmol) osthole and 0.531g (9.83mmol) sodium methoxide respectively and add them to a 100mL reaction tube, then add 30mL absolute ethanol, stir to dissolve, heat to reflux, TLC tracking, After 60 hours of reaction, the solvent was concentrated by rotary evaporation, 20 mL of distilled water was added to the concentrated solution, extracted with ethyl acetate, the aqueous layer was adjusted to pH=2-3 with 1.0 mol / L HCl, extracted with ethyl acetate, and dried over anhydrous magnesium sulfate. , filtered with suction, concentrated the solvent, separated and purified by silica gel column chromatography to obtain intermediate I.
[0025] Take 0.040g (0.15mmol) of Intermediate I, 0.046g (0.31mmol) of HOBT, 0.06mL (0.46mmol) of triethylamine and 10mL of anhydrous dichloromethane into a 50mL three-neck flask, under ice bath, dropwise add 5mL of EDCI 0.058 g (0.31 mmol) dissolved in anhydrous dichloromethane was stirred for reaction...
Embodiment 2
[0027] Preparation of compound II-2: Weigh 0.400g (1.64mmol) osthole and 0.531g (9.83mmol) sodium methoxide respectively and add them to a 100mL reaction tube, then add 30mL anhydrous ethanol, stir to dissolve, heat to reflux, TLC tracking, After 60 hours of reaction, the solvent was concentrated by rotary evaporation, 20 mL of distilled water was added to the concentrated solution, extracted with ethyl acetate, the aqueous layer was adjusted to pH=2-3 with 1.0 mol / L HCl, extracted with ethyl acetate, and dried over anhydrous magnesium sulfate. , filtered with suction, concentrated the solvent, separated and purified by silica gel column chromatography to obtain intermediate I.
[0028] Take 0.040g (0.15mmol) of intermediate I, 0.045g (0.30mmol) of HOBT, 0.06mL (0.46mmol) of triethylamine and 10mL of anhydrous dichloromethane into a 50mL three-neck flask, under ice bath, dropwise add 5mL of EDCI 0.057 g (0.301 mmol) dissolved in anhydrous dichloromethane was stirred and the re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com