Modulators of fpr1 and methods of use thereof
A C1-C6, C1-C4 technology, applied to medical preparations containing active ingredients, pharmaceutical formulas, metabolic diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0193] Example 1. Synthesis of Exemplary Compounds
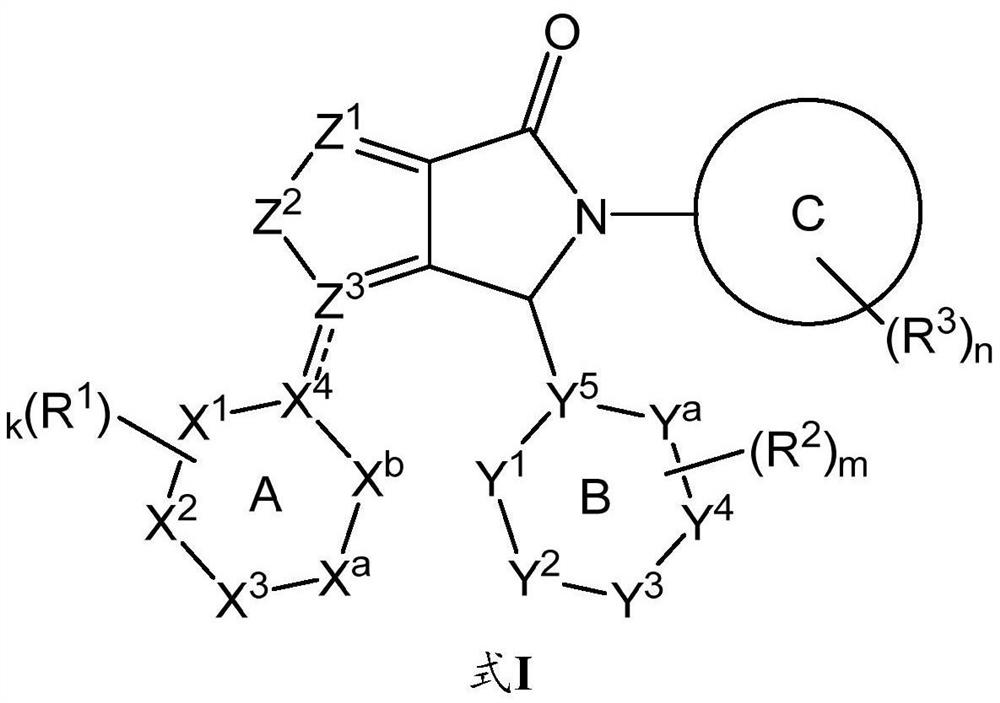
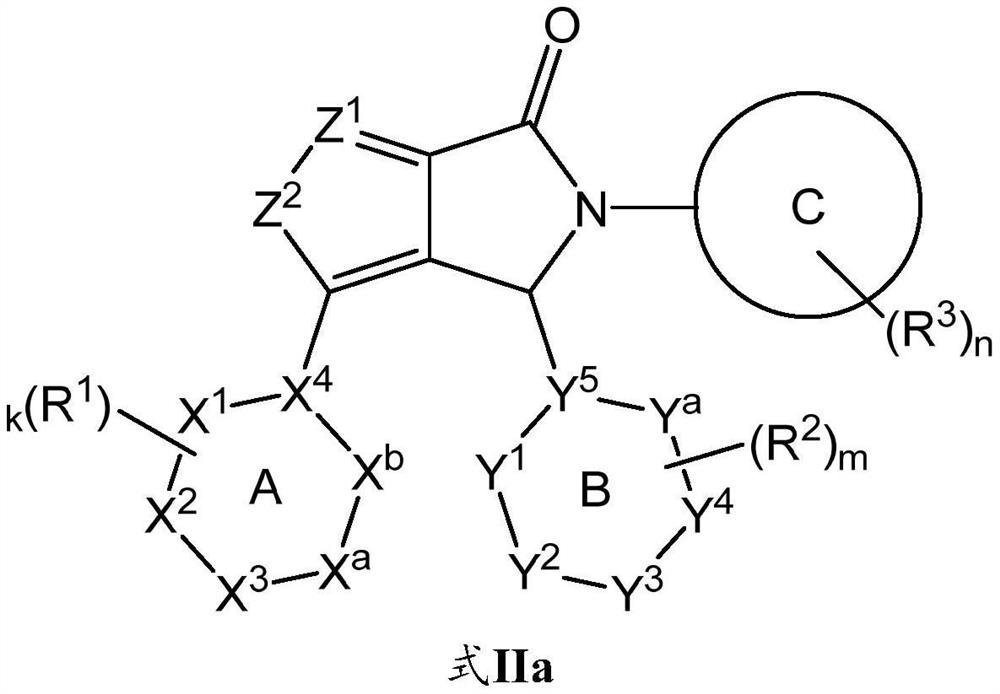
[0194] Compounds selected from the group consisting of formulae I, IIa, IIb, III, IV, V, VI and VII, compounds 1 to 4, their tautomerism can be prepared according to standard chemical practice or as described herein (including the synthetic schemes below) and in The compounds of the present disclosure are made as described in the description of the isomers, deuterated derivatives of the compounds or the tautomers, or pharmaceutically acceptable salts of the foregoing compounds.
[0195] Using compounds 1 and 2 as representative examples, methods for preparing compounds of formula I include general reaction procedures as described in Scheme 1 .
[0196] Compounds 1 and 2
[0197] Racemic-3-(2-hydroxy-5-methylphenyl)-5-(tetrahydro-2H-pyran-4-yl)-4-(4-(trifluoromethyl)phenyl)- 4,5-Dihydro-6H-pyrrolo[3,4-c]isoxazol-6-one
[0198] (Compound 1)
[0199] Racemic-3-(2-hydroxy-5-methylphenyl)-5-(tetrahydro-2H-pyran-4-yl)-4-(4-(tr...
Embodiment 2
[0229] Example 2. In vitro assay for detection and measurement of modulation of FPR1-mediated calcium signaling by compounds 1 to 4
[0230] As shown in the illustrative examples in Table 2, the effects of compounds of the present disclosure on modulating FPR1-mediated cellular signaling were measured by monitoring changes in cellular calcium levels. The following ranking criteria were used to unambiguously report the dose-response of the examples shown: ***(IC 50 ≤100nM);**(IC 50 ≥100 to ≤1000nM); *(IC 50 ≥1000 to ≤10,000 nM); N.D. - not detected.
[0231] Expression of human or mouse FPR1 in 293T cells
[0232] The coding DNA sequences (CDS) of human FPR1 (NM_001193306) and mouse FPR1 (NM_013521) were cloned and inserted into the lentiviral vector GV367 (vector information: http: / / www.genechem.com. cn / index / supports / tool_search.html?keywords=GV367). 293T cells were cultured in H-DMEM supplemented with 10% FBS and 1% penicillin-streptomycin (PS) at 37°C in a 5% CO2 incub...
Embodiment 3
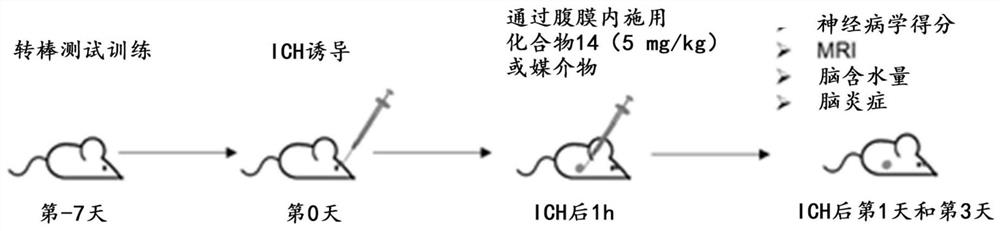
[0237] Example 3. In vivo preclinical efficacy of intracerebral hemorrhage (ICH) mouse model
[0238] The efficacy of the compounds of the present disclosure in protecting brain damage and improving brain function following stroke and / or brain injury was demonstrated in experiments described below using Compound 1 as a representative compound in an experimental ICH mouse model.
[0239] Preparation of experimental mouse ICH model
[0240] using a mouse model of intracerebral hemorrhage (ICH) to illustrate the protective benefit of compounds of the present disclosure, and figure 1 The procedure to prepare this model is depicted. ICH was induced in C57 B / L6 male mice by injection of autologous blood or collagenase as previously described (Lauer et al., Circulation 124:1654-1662 (2011); Rynkowski et al., Nat. Protoc. 3:122- 128 (2008)). Mice were anesthetized using isoflurane inhalation and fixed on a stereotaxic frame. A hole was drilled on the right side of the skull 2.3 mm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com