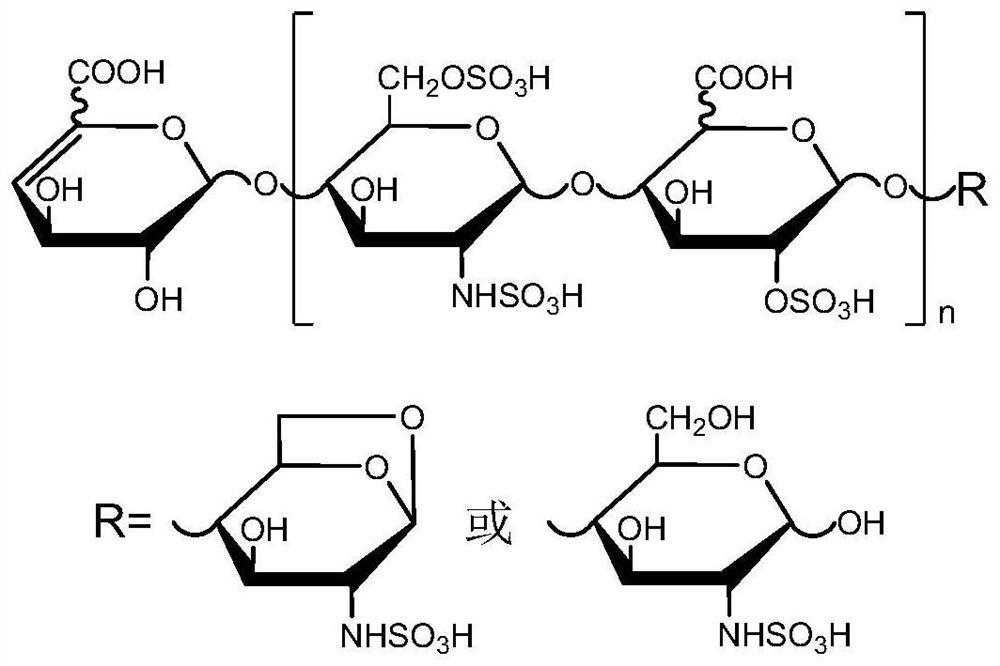
Two-dimensional liquid chromatography-mass spectrometry method for mass analysis of low-molecular-weight heparin
A low molecular weight heparin, two-dimensional liquid phase technology, applied in the field of drug analysis, can solve the problems of difficult structure consistency evaluation, complex structure, lack of analysis methods, etc., to achieve excellent separation ability and meet the needs of rapid analysis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] 1. Preparation of Enoxaparin Sodium Oligosaccharide by Column Chromatography
[0051] Using a protein purifier, the column is packed with size exclusion ( Gel) for column chromatography. Enoxaparin sodium was loaded at a concentration of 500 mg / ml, dissolved in a NaCl solution with a concentration of 0.5 mol / L in the mobile phase, and the samples were separated by isocratic elution at a flow rate of 5 ml / min. Using a UV detector, the detection wavelength was 232 nm.
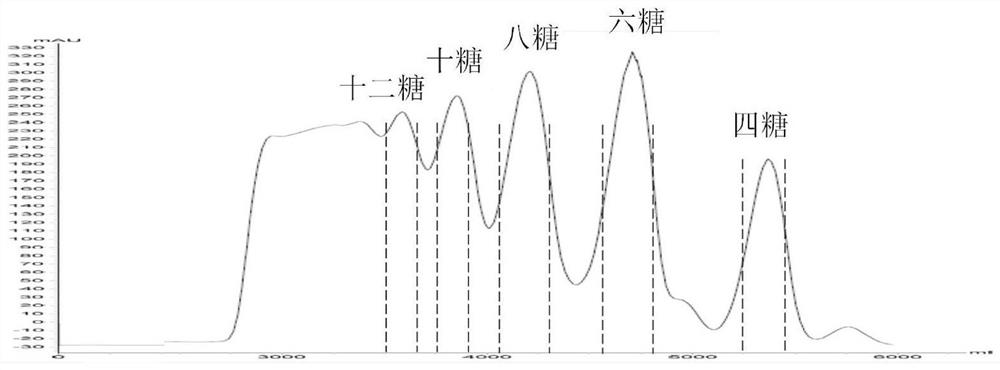
[0052] see figure 2 , figure 2 It is the low molecular weight heparin column chromatography chromatogram of the present invention. like figure 2 As shown, the oligosaccharides are collected by taking the half-peak width above the online detection, and the collected oligosaccharides have a single degree of polymerization. After concentration by rotary evaporation, the salt was removed by gel column chromatography and freeze-dried. 35.6 mg of enoxaparin sodium tetrasaccharide, 45.1 mg of hexasacch...
Embodiment 2
[0076] 1. Preparation of bemiparin sodium oligosaccharide by column chromatography
[0077] Using a protein purifier, the column is packed with size exclusion ( Gel) for column chromatography. Bemiparin sodium was loaded at a concentration of 500 mg / ml, dissolved in a NaCl solution with a concentration of 0.5 mol / L in the mobile phase, and the samples were separated by isocratic elution at a flow rate of 5 ml / min. Using a UV detector, the detection wavelength was 232 nm. The oligosaccharides are collected by taking the half-peak width above the online detection, and the collected oligosaccharides have a single degree of polymerization. After concentration by rotary evaporation, the salt was removed by gel column chromatography and freeze-dried. The single polymerized bemiparin sodium tetrasaccharide 19.7 mg, the hexasaccharide 23.1 mg, the octasaccharide 28.0 mg, the deca sugar 22.4 mg, and the dodecose 18.1 mg were prepared.
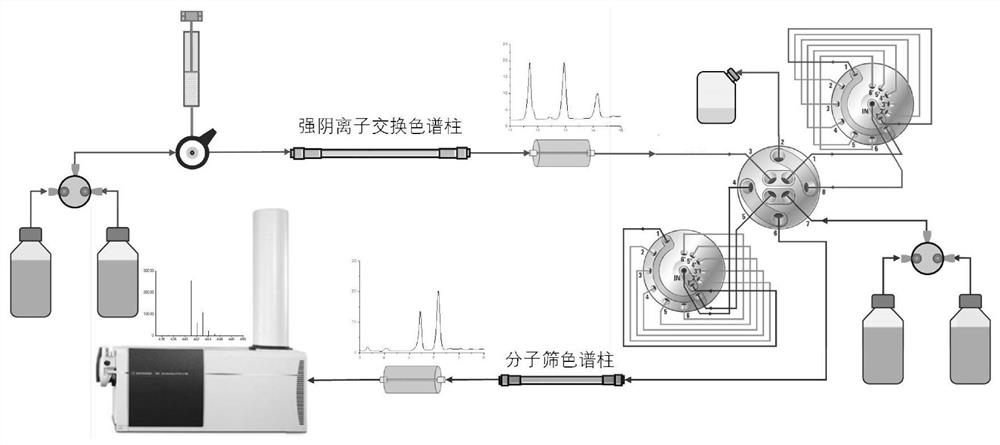
[0078] 2. SAX-SEC-MS qualitative experiment ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com