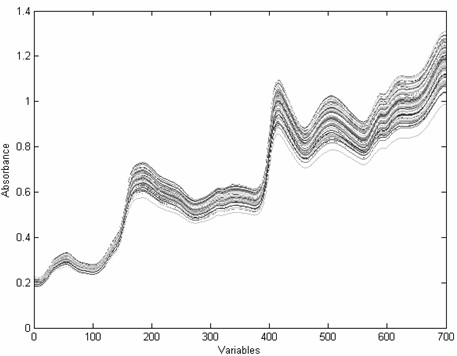
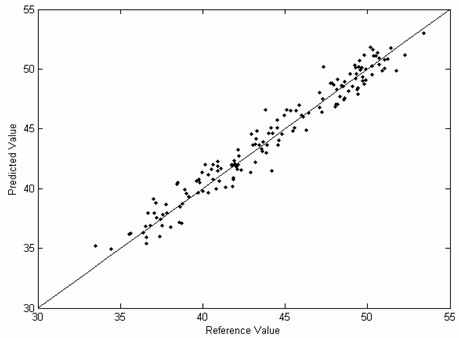
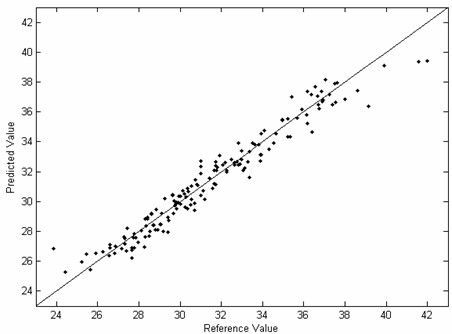
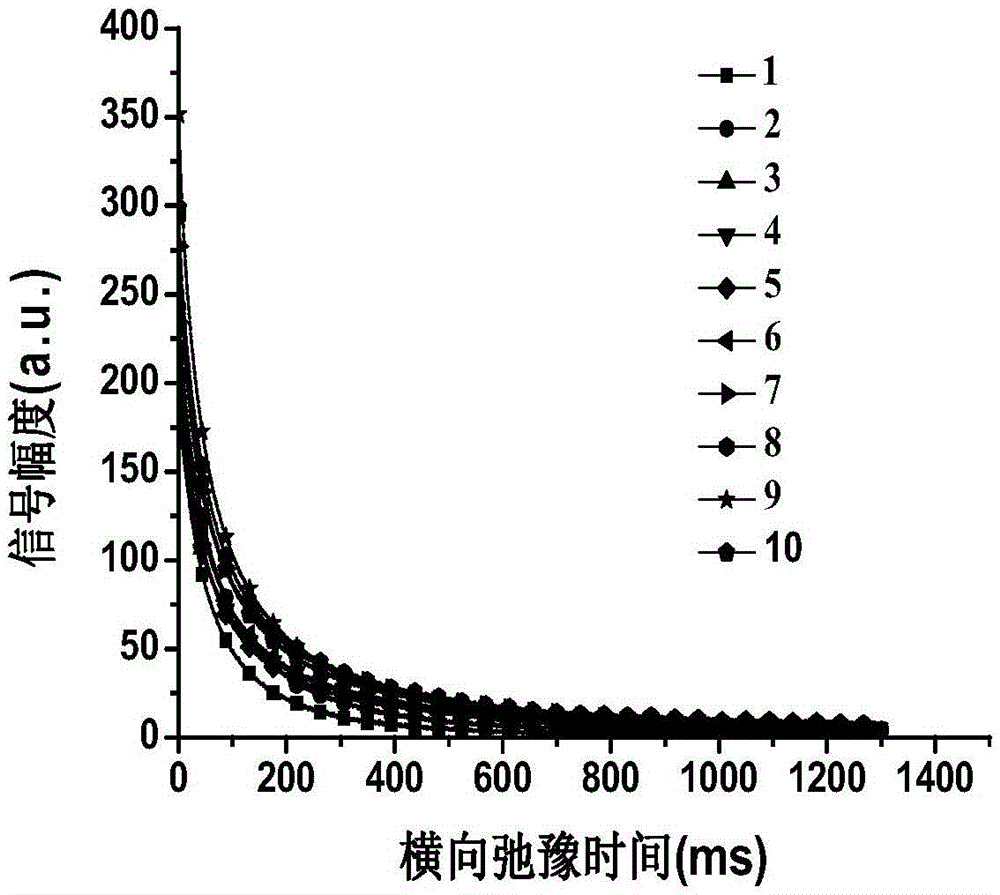
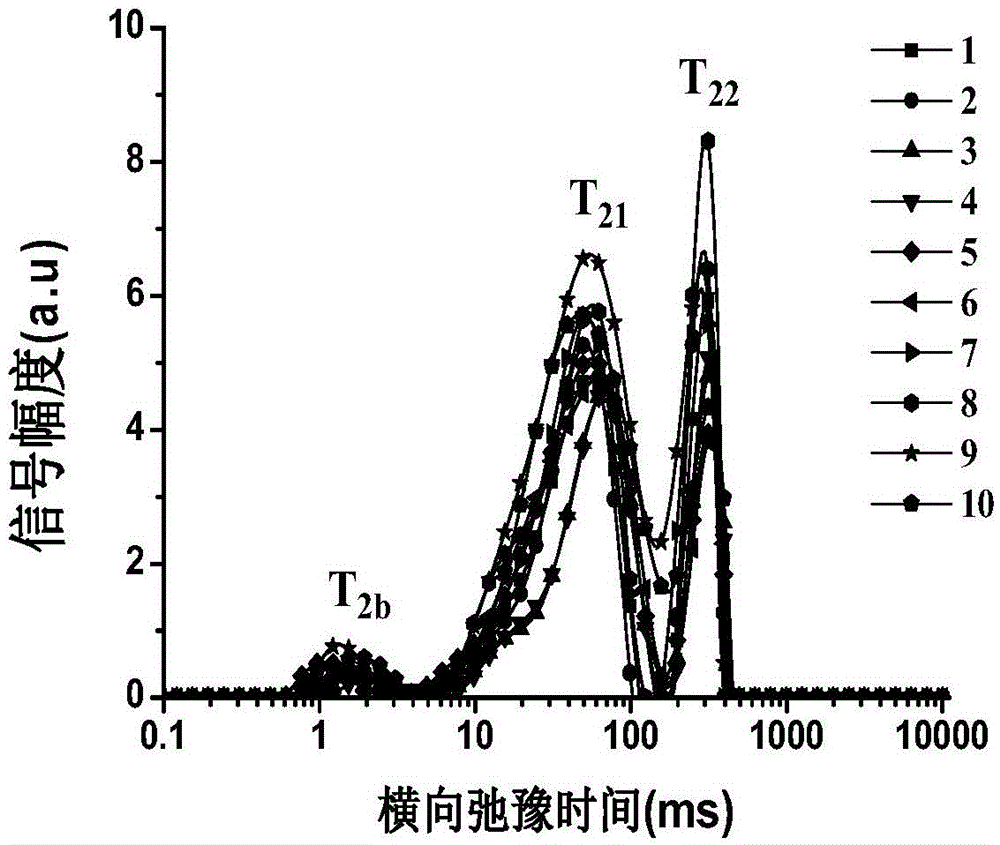
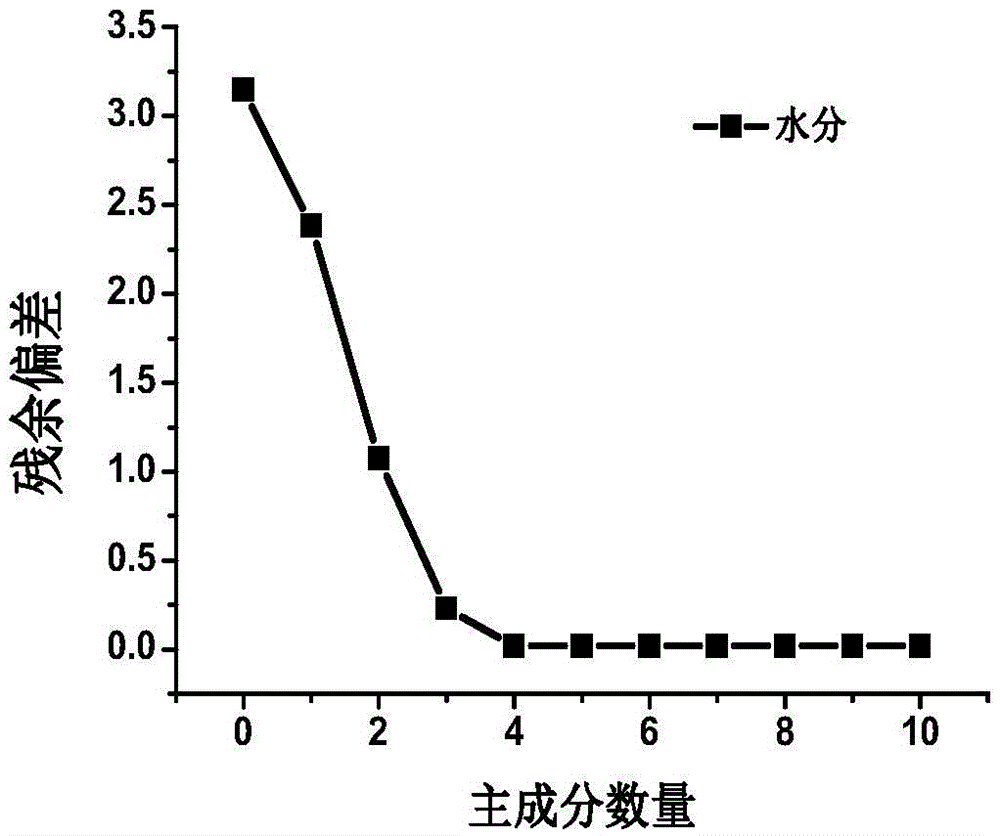
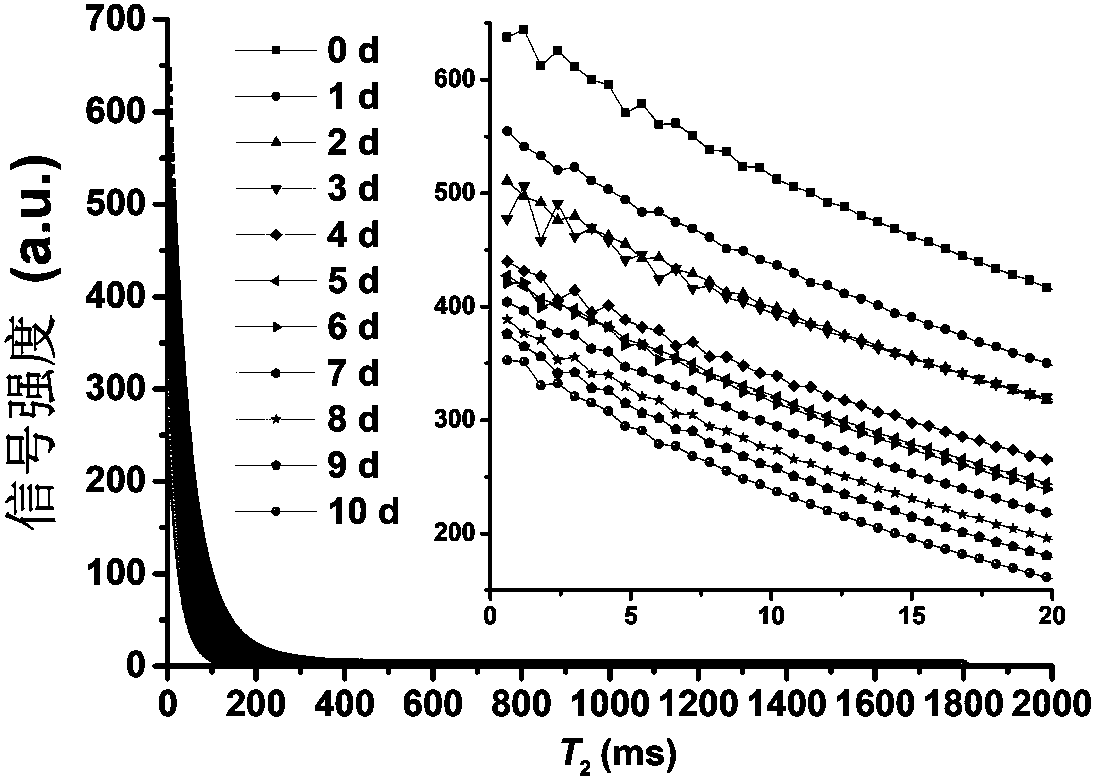
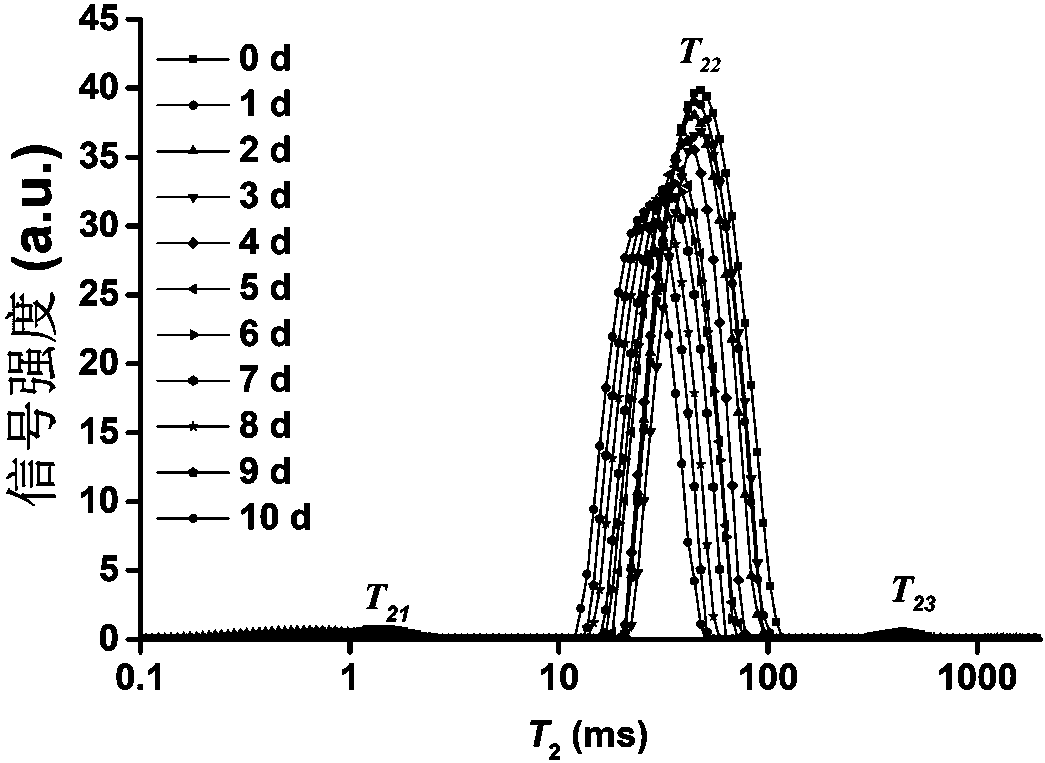
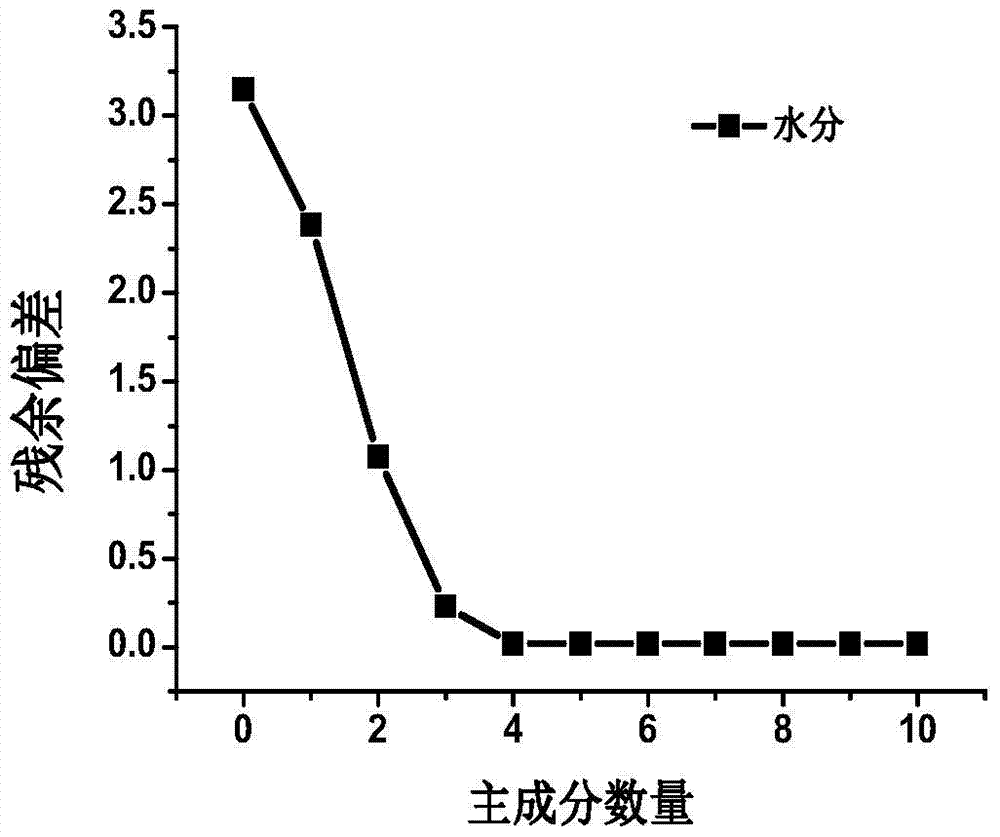
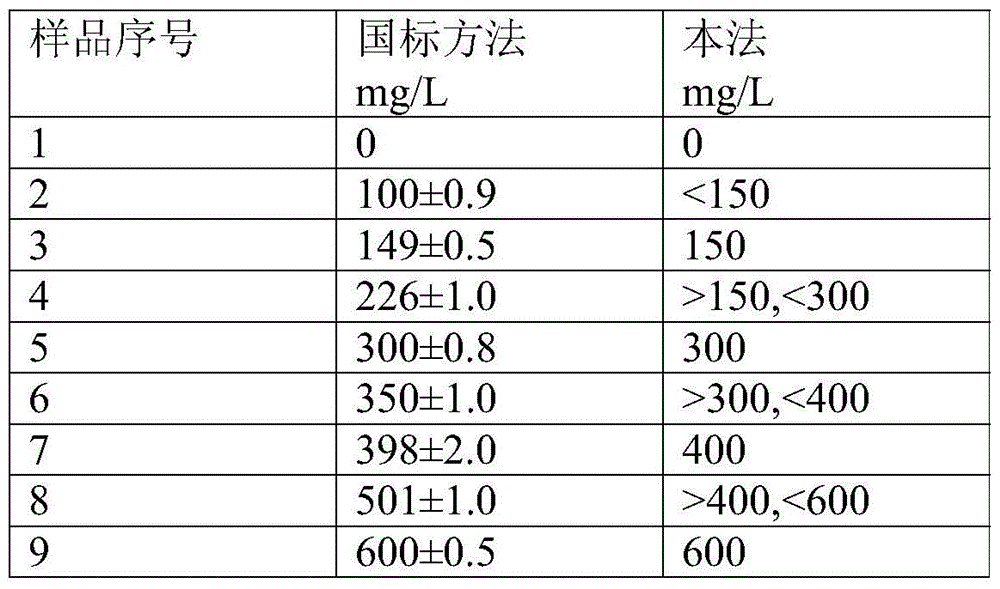
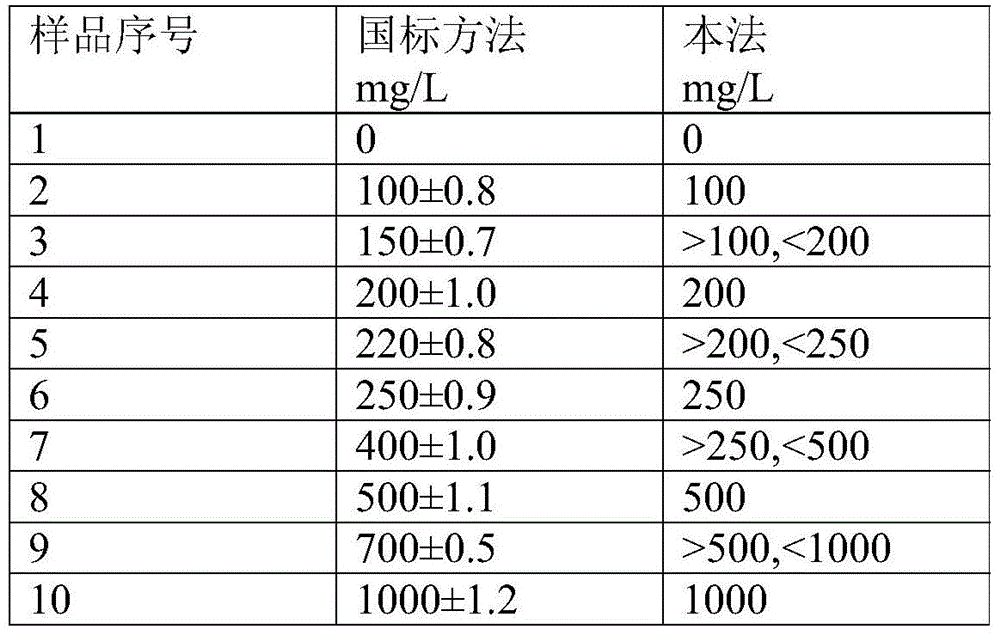
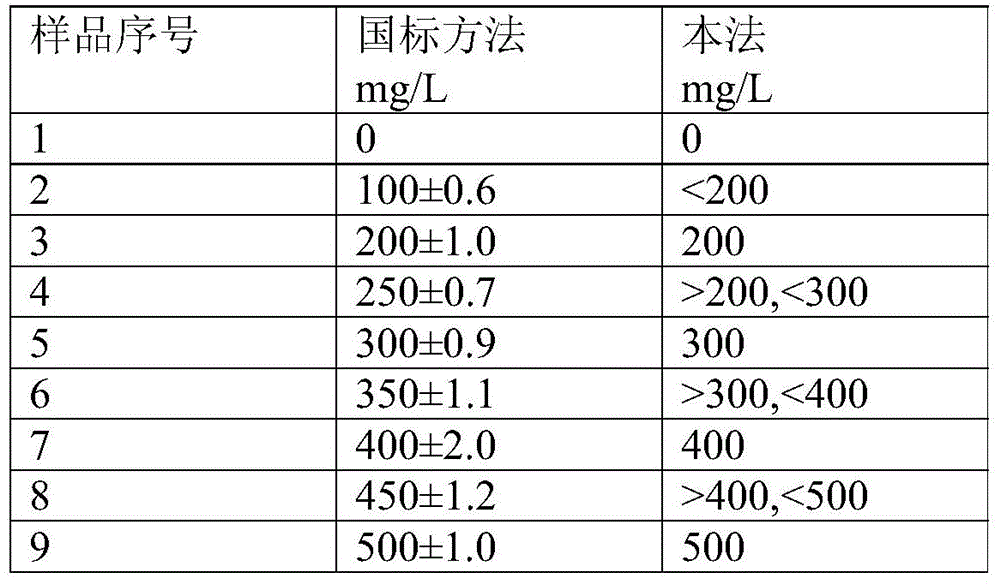
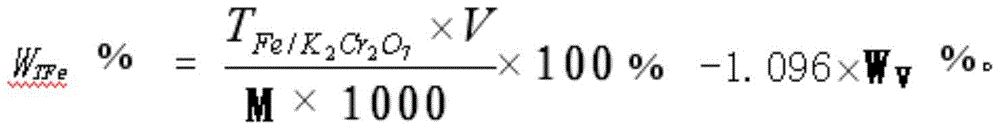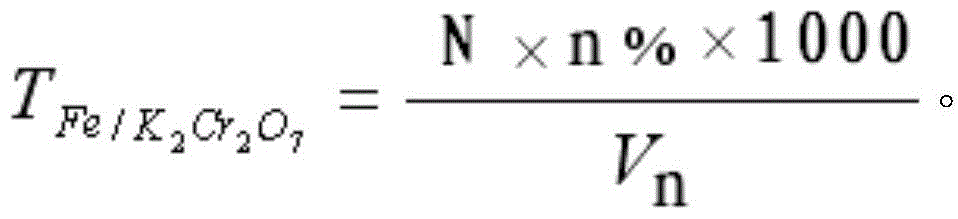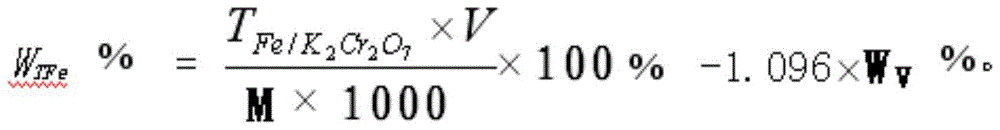Patents
Literature
34results about How to "Meet the needs of rapid analysis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A method for rapid and non-destructive analysis of nutritional quality of whole cottonseed based on near-infrared spectroscopy
InactiveCN102279168AEliminate distractionsImprove signal-to-noise ratioColor/spectral properties measurementsNondestructive analysisNutrition
The invention provides a novel fast and non-destructive analysis method for the nutritional quality of cottonseed. The method comprises the following steps: 1) selecting whole cottonseeds of representative cotton varieties as experimental samples; 2) using a near-infrared spectrometer to collect near-infrared spectral data of whole cottonseeds, and the spectral scanning range is 800-2500nm; 3) using the national standard method Accurately analyze the nutritional quality of cottonseed; 4) Preprocess the near-infrared spectrum data; 5) Select spectral variables to eliminate non-informative variables; 6) Use the multivariate correction method to establish the near-infrared spectrum of whole cottonseed and the nutritional value of whole cottonseed 7) Performance evaluation of the calibration model; 8) Collect near-infrared spectral data of the whole cottonseed to be tested, and use the calibration model to predict its nutritional quality after preprocessing the spectral data. The invention has fast analysis speed, does not destroy samples, does not require sample pretreatment, has high detection accuracy, and can analyze various nutritional quality components in cotton seeds at the same time, and provides a convenient, fast and efficient analysis method for cotton breeding and cotton seed inspection.
Owner:ZHEJIANG UNIV
Method for detecting water content and fat content in abalone through low-field nuclear magnetic resonance technology
ActiveCN105606637AEasy to operateMeet the needs of rapid analysisAnalysis using nuclear magnetic resonanceLow field nuclear magnetic resonanceFat content
The invention provides a method for detecting the water content and fat content in abalone through a low-field nuclear magnetic resonance technology. The method comprises the following first step of collecting samples, the second step of carrying out low-field nuclear magnetic analysis on the sample, the third step of measuring the samples, the fourth step of establishing a model, the fifth step of evaluating the model, and the sixth step of measuring the water content and the fat content of the samples to be detected. By means of the method, the water content and fat content in abalone can be measured at the same time, detection is rapid and accurate and free of the influence of the surface feature of abalone, and abalone is not damaged in the measuring process.
Owner:DALIAN POLYTECHNIC UNIVERSITY
Low field nuclear magnetic resonance-applied frozen beef freshness nondestructive testing method
InactiveCN109444199AMeet the needs of rapid analysisBeef bustingAnalysis using nuclear magnetic resonanceNitrogenThiobarbituric acid
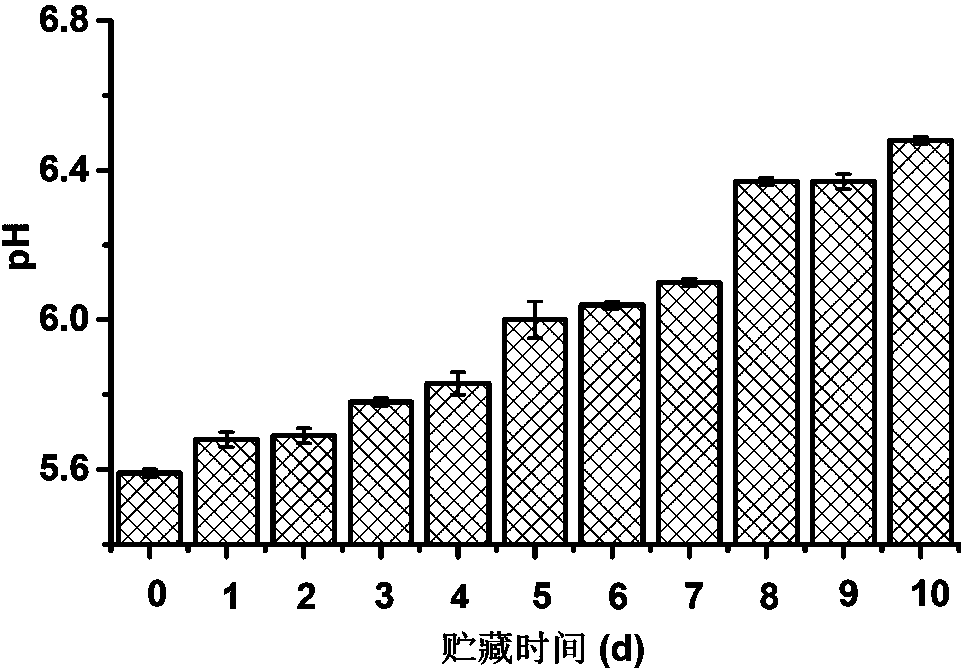
The invention discloses a low field nuclear magnetic resonance-applied frozen beef freshness nondestructive testing method. The low field nuclear magnetic resonance-applied frozen beef freshness nondestructive testing method comprises the following steps of (A) sample collection, (B) sample measurement, (C) sample low field nuclear magnetic analysis, (D) multiple freshness-related index measurement, (E) model establishment, (F) model assessment and (G) measurement of freshness of samples to be detected. By establishing a mathematical predication model reflecting fresh beef moisture content relaxation spectrum information and multiple indexes related to fresh beef freshness to accurately and reliably detect multiple indexes of an unknown fresh beef simultaneously such as thiobarbituric acidreactive substances (TBARS), volatile basic nitrogen (TVB-N) and pH, the low field nuclear magnetic resonance-applied frozen beef freshness nondestructive testing method can help predict the storagetime of the beef and achieve rapid and nondestructive detection and assessment of the freshness of the fresh beef. The low field nuclear magnetic resonance-applied frozen beef freshness nondestructivetesting method is rapid and accurate, avoids influence of beef surface properties, and during measurement, has no damage to the beef.
Owner:DALIAN POLYTECHNIC UNIVERSITY
Method for quickly analyzing content of rare earth in rock weathering crusts by using visible light-near infrared reflection spectrometer
InactiveCN109813665AImprove accuracyLow detection limitColor/spectral properties measurementsIon adsorptionOxide
The invention discloses a method for detecting the content of rare earth in rock weathering crusts by using a visible light-near infrared reflection spectrometer and analyzing data. The content of therare earth in outcrops, surface soil and drilling samples of the rock weathering crusts is tested by using the handheld visible light-near infrared reflection spectrometer, through specific sample pretreatment and data processing means, the interference of water, organic matter, ferric oxides and other components in the samples with the testing accuracy of the content of the rear earth is eliminated, the accuracy and the sensitivity of testing the content of the rear earth in the rock weathering crusts are improved, and the requirements of weathering crust ion adsorption type rare earth deposit ore prospecting and exploration work for quick analysis of the samples are met.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
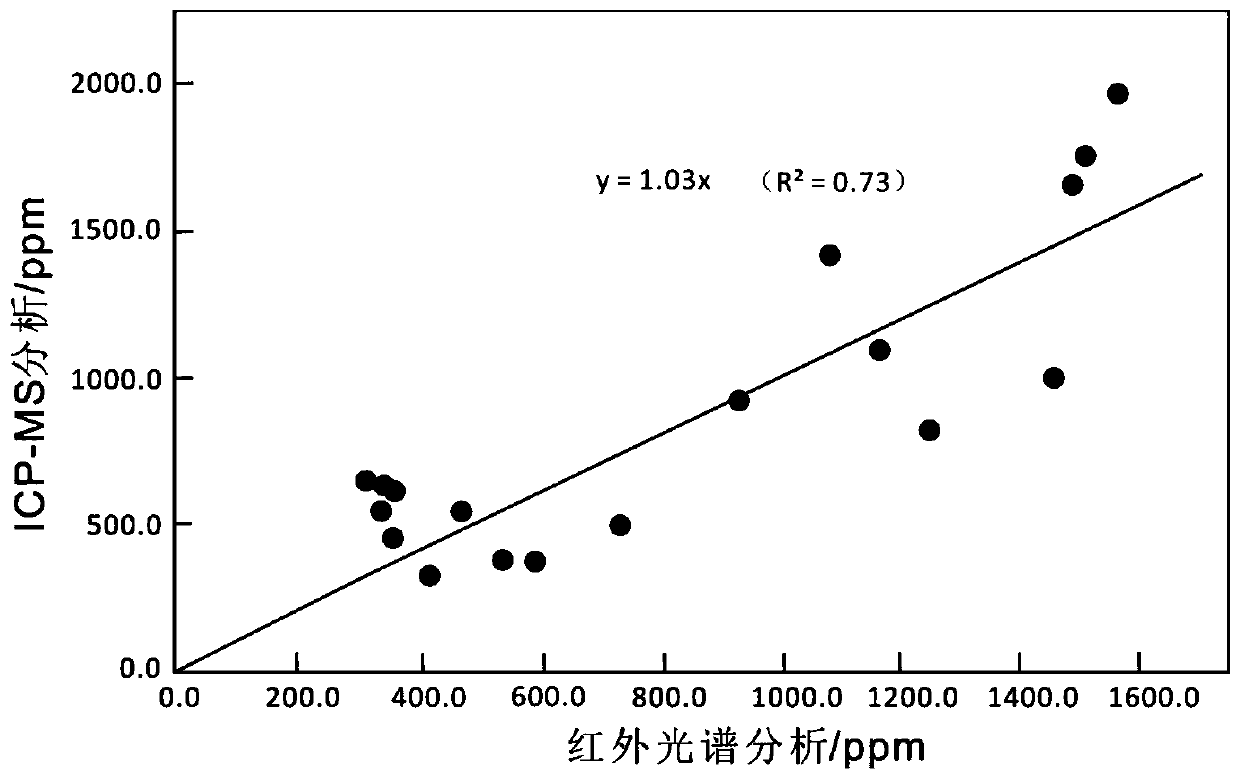
Method for quickly separating copper matrix for ICP-MS analysis of impurity elements in metal copper
InactiveCN110553887AEliminate distractionsMeet the test requirementsPreparing sample for investigationMaterial analysis by electric/magnetic meansElectrolysisManganese
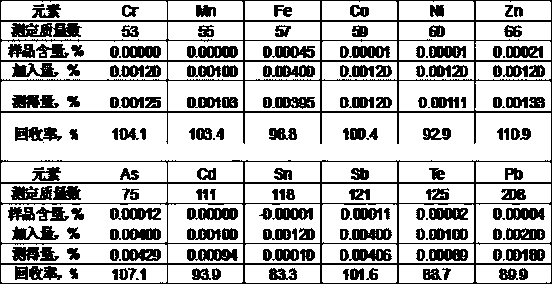
The invention relates to a method for quickly separating a copper matrix for ICP-MS analysis of impurity elements in metal copper, which comprises the following steps: digesting a metal copper sampleby using a mixed acid solution of nitric acid and sulfuric acid (a small amount) in a certain ratio, and removing the copper matrix by using a high-current quick electrolysis method to obtain a solution to be detected; and then adopting inductively coupled plasma mass spectrometry (ICP-MS) to realize rapid and simultaneous determination of twelve impurity elements of arsenic, antimony, tellurium,tin, lead, zinc, iron, nickel, cobalt, chromium, cadmium and manganese in the to-be-detected solution under certain instrument working conditions. Compared with the national standard GB / T 5121.28-2010, the method has the advantages that the separation effect is good, the matrix interference is eliminated, the twelve impurity elements of arsenic, antimony, tellurium, tin, lead, zinc, iron, nickel,cobalt, chromium, cadmium and manganese can be rapidly and simultaneously determined, the measurement time is short, and the requirement of rapid analysis of the twelve trace impurity elements in themetal copper is met.
Owner:北矿检测技术股份有限公司
Method for measuring volatile organic compound in tobacco material
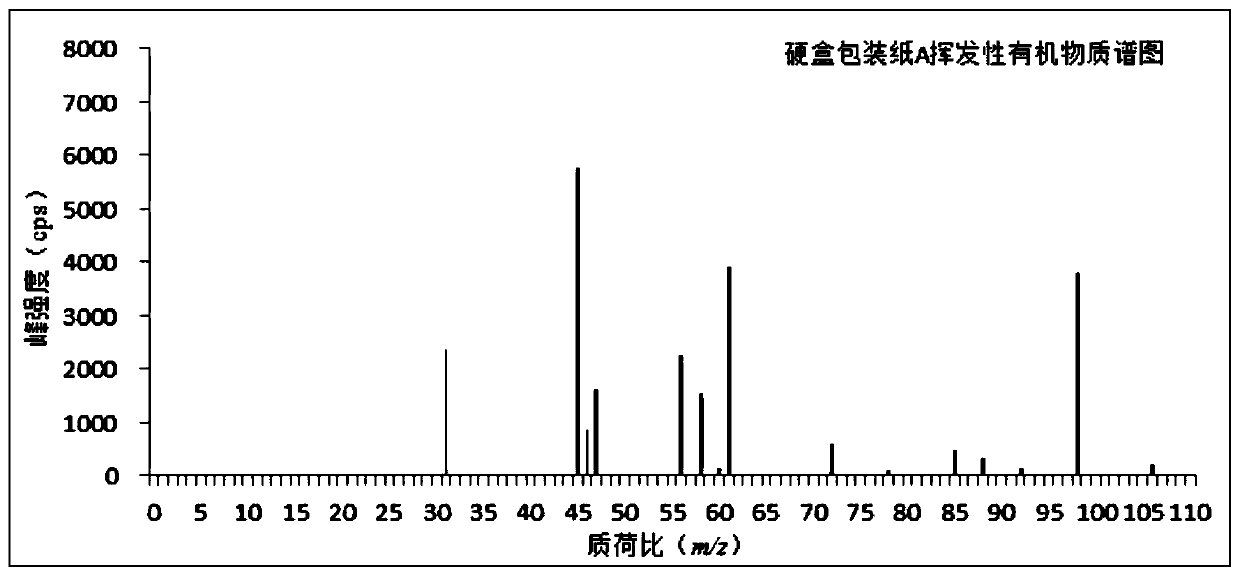
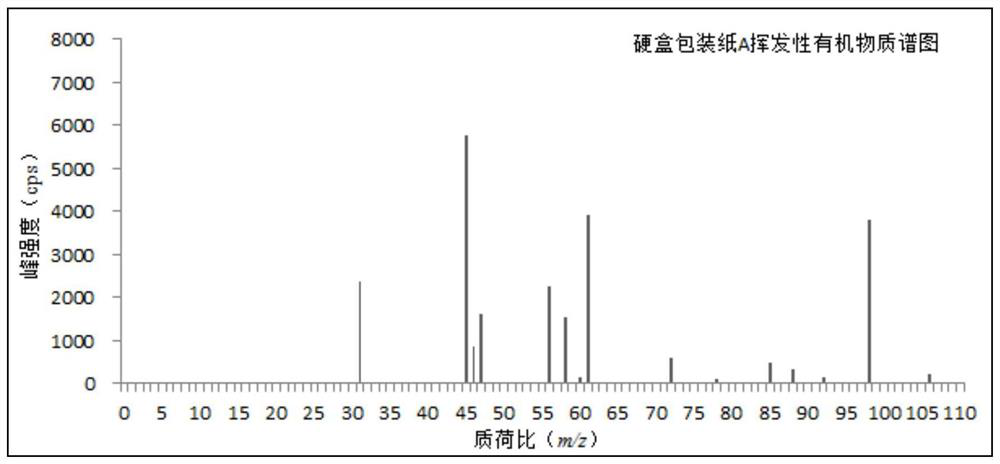
ActiveCN110763794AReduce processingRealize detectionComponent separationMass Spectrometry-Mass SpectrometryProcess engineering
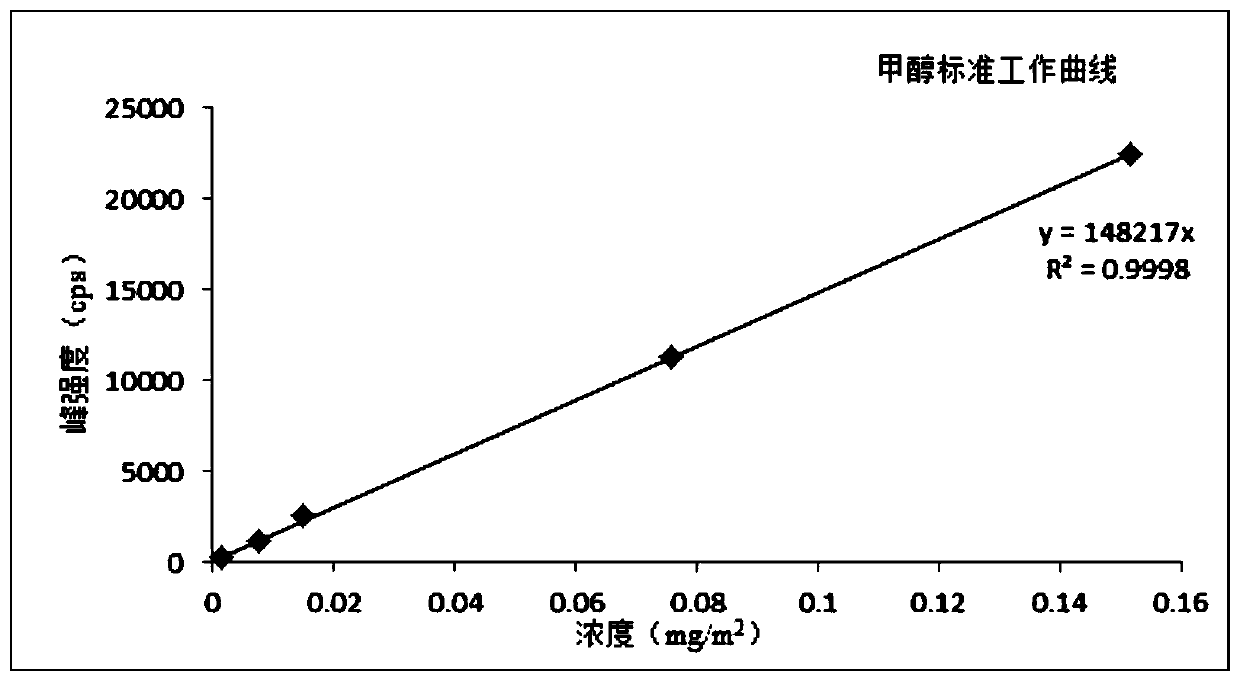
The invention discloses a method for measuring a volatile organic compound in a tobacco material. The method comprises the steps of using a headspace sampler and ion molecular reaction mass spectrometry (IMR-MS) in a combination way, collecting a volatile constituent in tobacco material paper (including cigarette paper, tipping paper, hard box package paper, soft box package paper, strip package paper, tobacco filter stick and filaments by a headspace sampler, analyzing by the IMR-MS, and achieving rapid and accurate measurement of the content of the volatile organic compound in the tobacco material by drawing a standard curve and by calculation. By the method, relatively-simple clean mass spectrum can be obtained, the qualitative and quantitative analysis of the volatile organic compoundis simplified, 19 types of volatile organic compound common in the tobacco material can be simultaneously, accurately and quantitatively detected, the advantage of rapid analysis is beneficial for achieving detection of a large amount of volatile samples, and the rapid analysis demand of a laboratory is satisfied.
Owner:CHINA TOBACCO YUNNAN IND
Semi-quantitative detection method of quaternary ammonium salt of water quality
ActiveCN104237219AGood effectMeet the needs of rapid analysisMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsEthylene diamineQuaternary ammonium cation
The invention discloses a semi-quantitative detection method of quaternary ammonium salt of water quality. The method comprises the following steps: sucking a water sample to be detected into a reaction tube, dripping a quaternary ammonium salt detection reagent into the reaction tube, uniformly shaking, then immediately carrying out color comparison between the color of the shaken solution and a testing standard color card, and determining the content of the quaternary ammonium salt by comparing the color of the shaken solution with the testing standard color card, wherein the quaternary ammonium salt detection reagent comprises titan yellow, ethylene diamine tetraacetic acid (EDTA), Thymol blue and sodium hydroxide, and is prepared by mixing the titan yellow, the EDTA, the Thymol blue and the sodium hydroxide according to the mass ratio of 100: (5-100): (20-200): (10-100). According to the method, the concentration of the quaternary ammonium salt in the water sample can be rapidly detected only by the quaternary ammonium salt detection reagent, the reaction tube and the testing standard color card; the method is simple to operate, can be operated in real time on site, and meets the demand of rapidly analyzing a great deal of samples.
Owner:ZHEJIANG UNIV
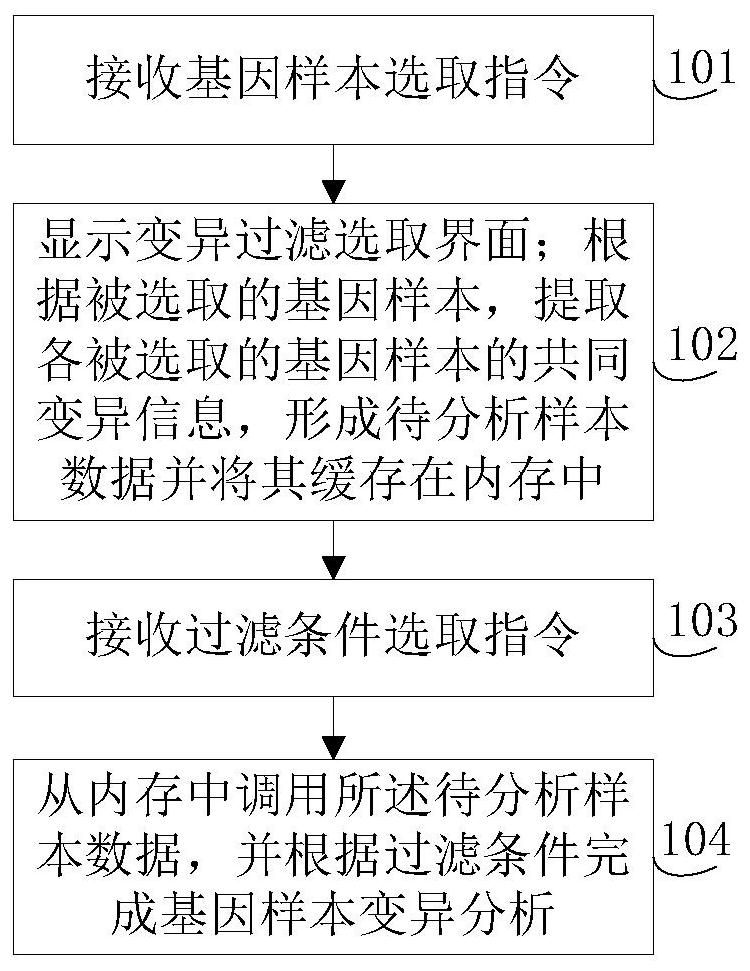
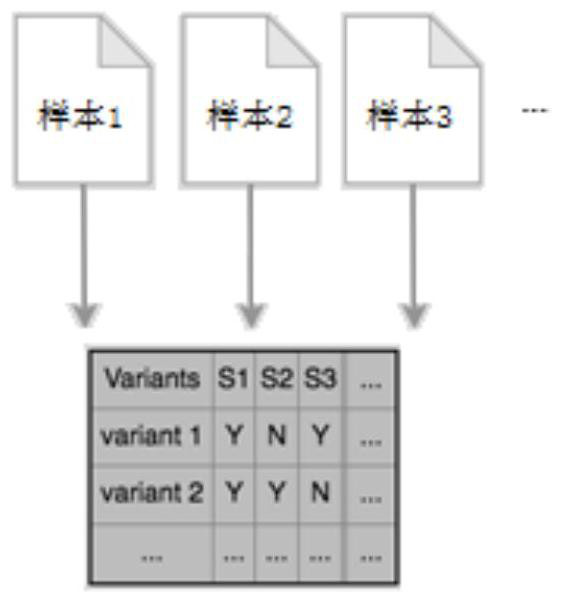
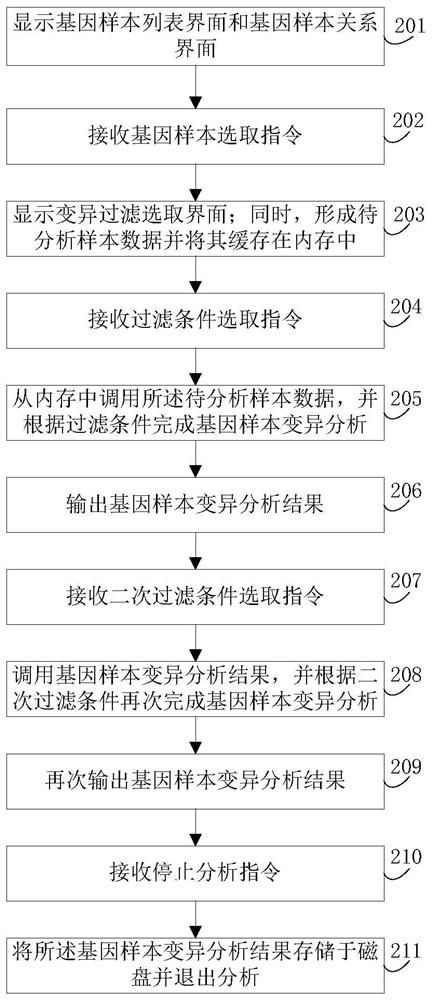
Gene-sample analysis method and electronic device
ActiveCN107480468ASmall footprintMeet the needs of rapid analysisHybridisationSpecial data processing applicationsAnalysis methodElectronic equipment
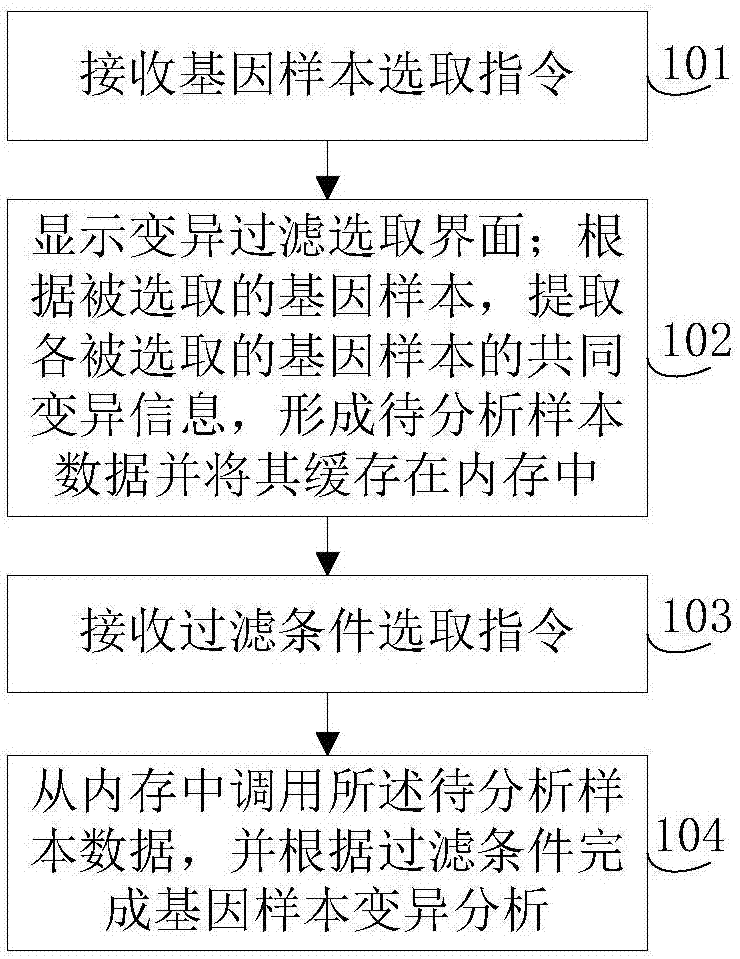
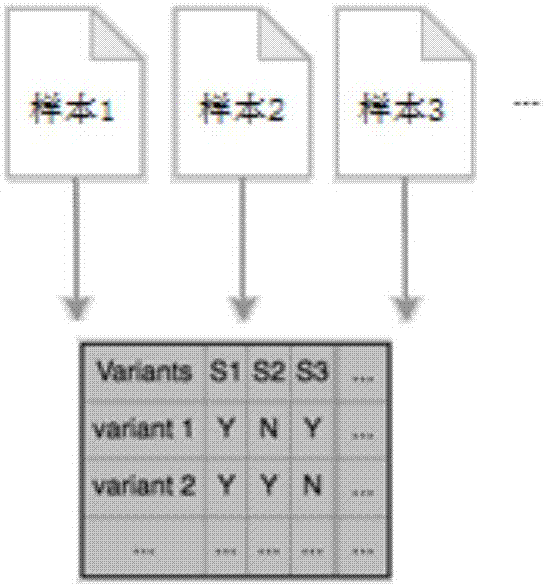
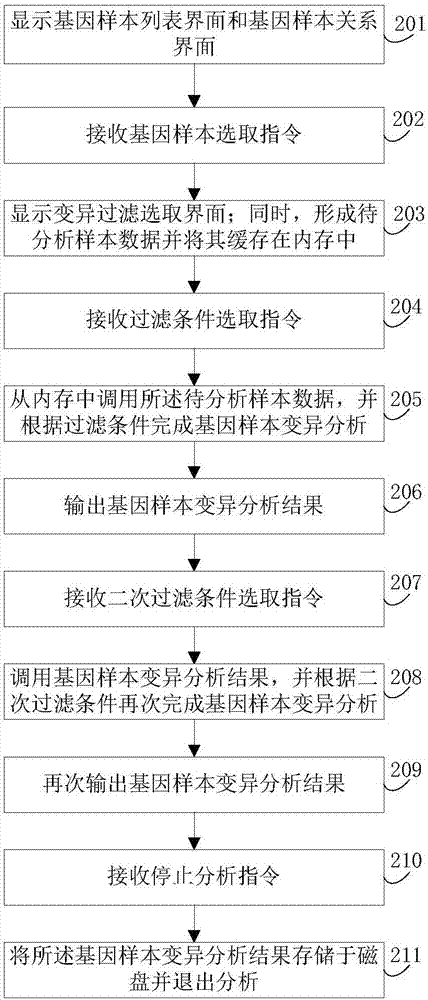
The invention discloses a gene-sample analysis method. The gene-sample analysis method includes the steps that gene-sample selection instructions are received; a variation filter selection interface is displayed; meanwhile, according to selected gene samples, common variation information of all the selected gene samples is extracted, and to-be-analyzed sample data is formed and cached in a memory; filter-condition selection instructions are received; the to-be-analyzed sample data is called from the memory, and gene-sample variation analysis is completed according to filter conditions. The invention discloses an electronic device with the gene-sample analysis method. By means of the gene-sample analysis method and the electronic device, the response speed of gene-sample analysis can be increased.
Owner:UNITED ELECTRONICS
Gridding-based point cloud data retrieval method
PendingCN113190723AAvoid traversalAvoid efficiencyOther databases queryingOther databases clustering/classificationCluster algorithmPoint cloud
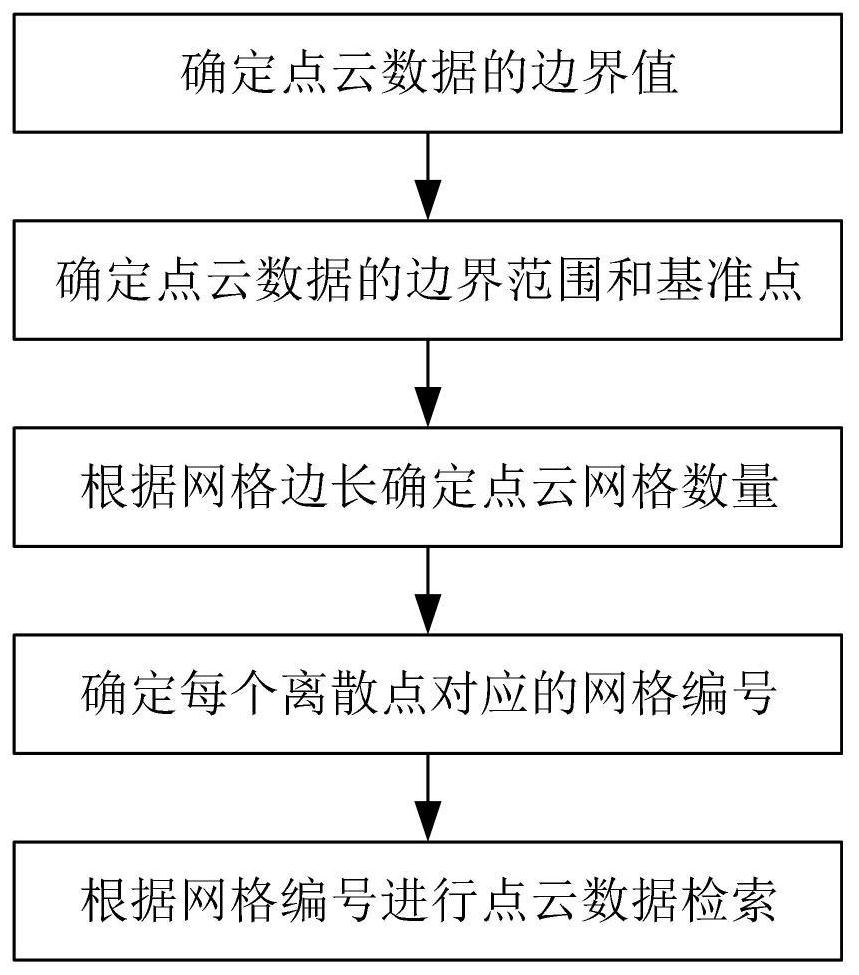
The invention relates to a gridding-based point cloud data retrieval method. The method comprises the following steps: determining a boundary value of point cloud data; determining a boundary range and a reference point of the point cloud data according to the boundary value; dividing the point cloud data into point cloud grids according to the boundary range and a preset grid side length, and determining the number of the point cloud grids; determining index numbers of discrete points in the point cloud grids according to the reference points and the number of the point cloud grids; and retrieving the point cloud data according to a preset retrieval method and the index number. Through the steps, the point cloud data formed by a large number of discrete points are gridded, and retrieval is performed according to the adjacent clustering algorithm by taking the gridded point cloud grid as a unit in the point cloud data retrieval process, so that the problem of low efficiency caused by traversing the point cloud data in an existing mode is avoided, the data retrieval efficiency is improved, and the requirement on rapid analysis of the point cloud data is met.
Owner:YUNNAN POWER GRID CO LTD ELECTRIC POWER RES INST
Device and method for analyzing Sr-90 in reactor decommissioned stainless steel material
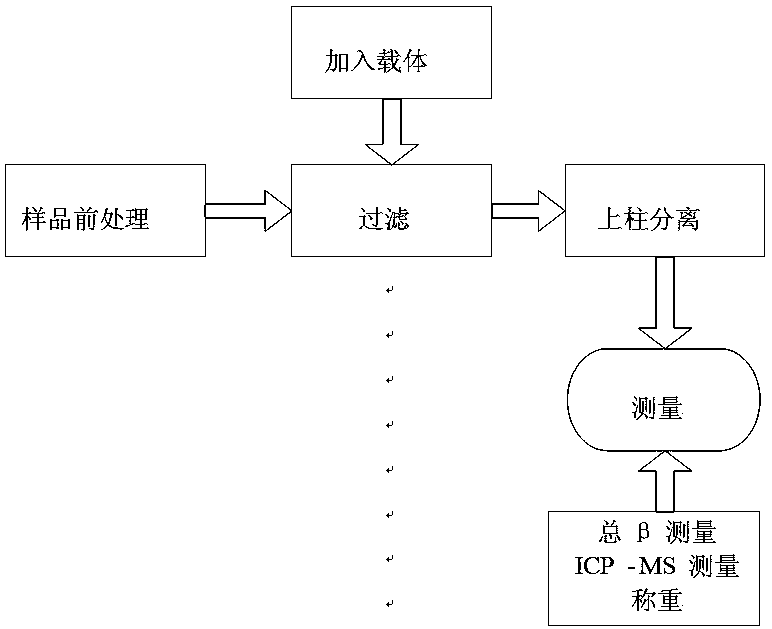
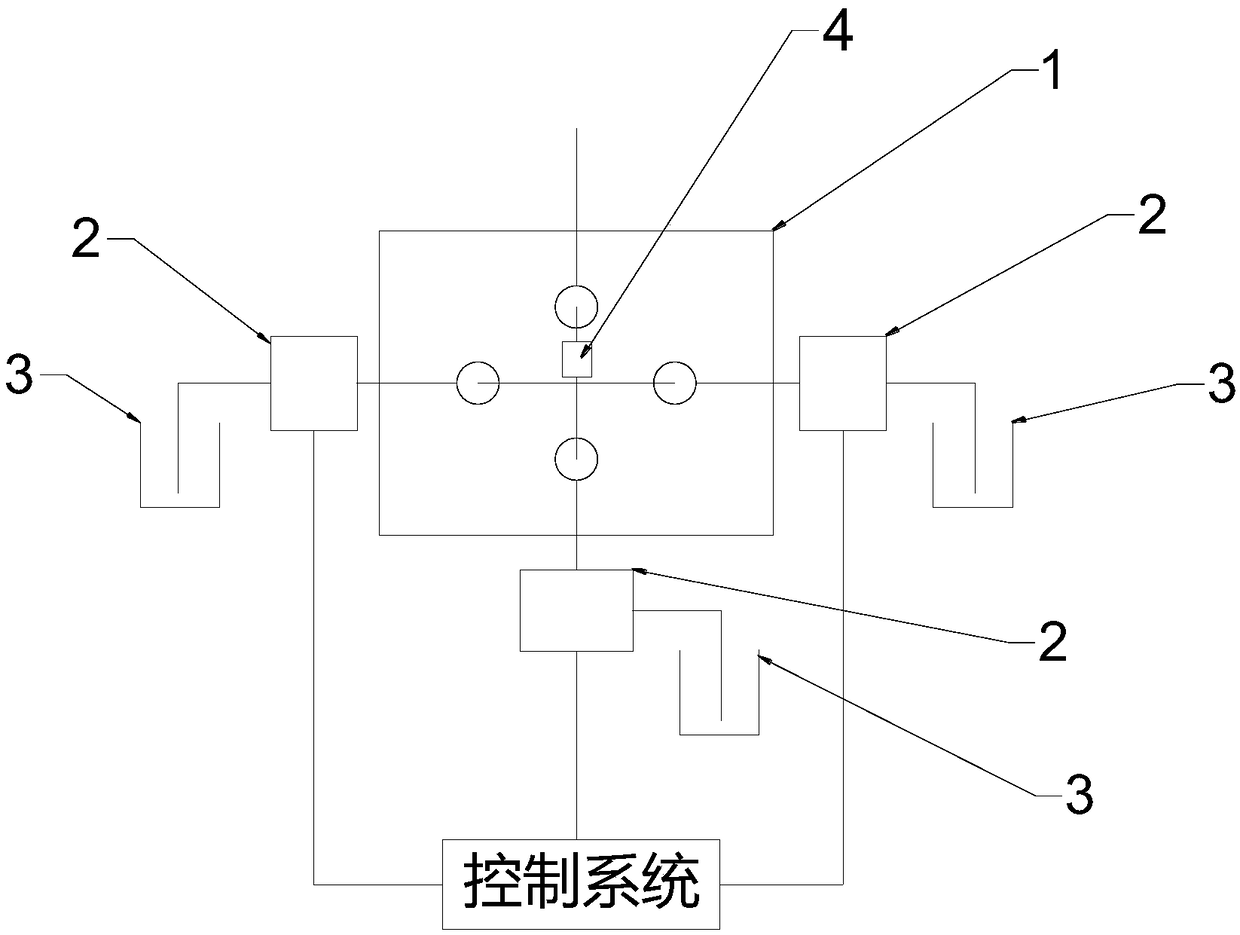
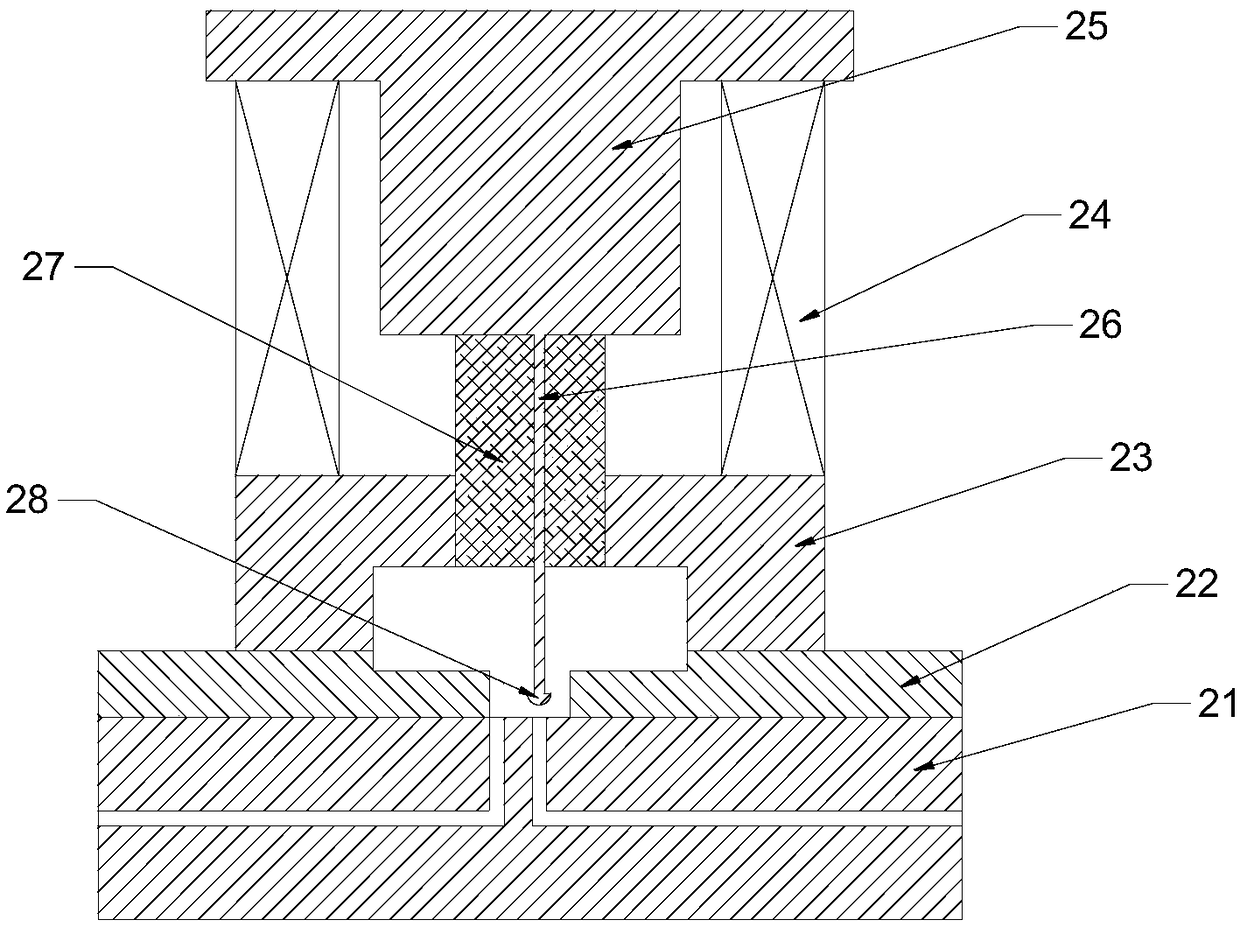
InactiveCN109324339AMeet the needs of rapid analysisReduce usageX/gamma/cosmic radiation measurmentActivity concentrationContact time
The invention discloses a device and method for analyzing Sr-90 in a reactor decommissioned stainless steel material. The device is divided into a separating unit and a measuring unit; the separatingunit achieves rapid separation of the Sr-90 and a daughter Y-90 of the Sr-90; the measuring unit measures the activity concentration of the separated and purified daughter Y-90 of the Sr-90; and thenthe activity concentration of the Sr-90 is calculated according to the Y-90. According to the method, the treating time is short, and the requirements of rapid separation and analysis are met; meanwhile, the analysis process is simplified and controllable, the using amount of chemical reagents and the contact time of workers are reduced, and the adverse effects of hazards of radioactive reagents and the chemical reagents on occupational health of the workers are avoided; and moreover, according to the principle of selective adsorption separation, the daughter Y-90 of the Sr-90 is efficiently adsorbed and desorbed, and the high recovery efficiency is guaranteed. The method has the advantages of short separation time, simple process, high recovery rate, good system stability, long service life and the like, and the needs of rapid analysis of the Sr-90 in the stainless steel material in a reactor can be met.
Owner:INST OF NUCLEAR PHYSICS & CHEM CHINA ACADEMY OF
Online dilution device
PendingCN108854837ARealize in-line dilution and mixingHigh degree of automationTransportation and packagingMixer accessoriesHigh concentrationControl system
The invention discloses an online dilution device, comprising a dilution device body and a control system, wherein the dilution device body comprises a fixed base and at least one proportional distributor; at least one liquid inlet pipeline and at least one liquid outlet pipeline are formed on the fixed base; an inlet of the proportional distributor is connected to a liquid inlet container througha pipeline, and an outlet of the proportional distributor is in communication with the liquid inlet pipeline of the fixed base; the liquid outlet pipeline is connected with a flow injection analyzer;the control system is connected to the proportional distributor by signals. The online dilution device can realize the online preparation of a standard curve based on the structure above, perform on-line dilution of high concentration samples with dilution ratio of 1 to 200 times, dilute and mix a sample and 12 kinds of reagents on line and work with the flow injection analyzer together without interference, has not only a high degree of automation, but also high work efficiency, and thus meets the needs of rapid analysis.
Owner:BEIJING BAODE INSTR CO LTD
Method for quickly determining content of barium in nickel salt solution
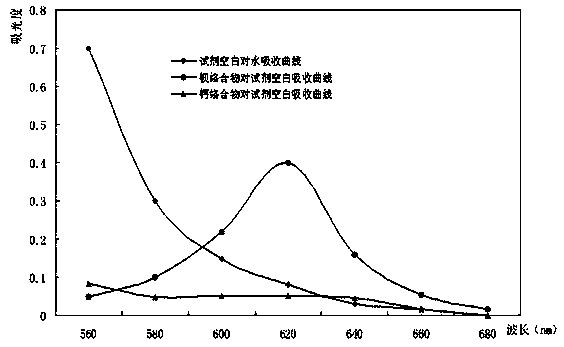
InactiveCN109142246ALarge amount allowedCo-precipitation has little effectColor/spectral properties measurementsMicrogramNickel salt
The invention discloses a method for quickly determining the content of barium in a nickel salt solution. In 0.12 mol / L of hydrochloric acid medium, tribromoarsenazo and barium can form a stable purple complex, and according to the spectrophotometric analysis principle, accurate determination of the content of the barium in a nickel chloride solution or a nickel nitrate solution is completed. Themethod comprises the specific steps that 5.0 mL of 0.6 mol / L hydrochloric acid solution and 2.0 mL of 50 g / L ascorbic acid solution are added into the pretreated nickel chloride or nickel nitrate solution in a 25 mL volumetric flask in sequence and mixed evenly, then 5.0 mL of 1g / L tribromoarsenazo solution is added, and the volume is metered; with the reagent blank as a reference, the absorbancyis measured at the wave length of 620 nm, and according to the absorbancy value, the content of the barium in the to-be-measured solution is obtained on a working curve. By means of the method, the content of the barium is within the range from 0 microgram / 25 mL to 50 micrograms / 25 mL and accords with the Beer's law, and the apparent molar absorption coefficient epsilon is 4.64*10<4>Lmol<-1>cm<-1>; the recycling rate is 95-103%, RSD is smaller than 10%, the method is stable, reliable, quick and simple, and requirements for quick analysis of the barium in the nickel chloride and nickel nitrateproduction process can be met.
Owner:JINCHUAN GROUP LIMITED +1
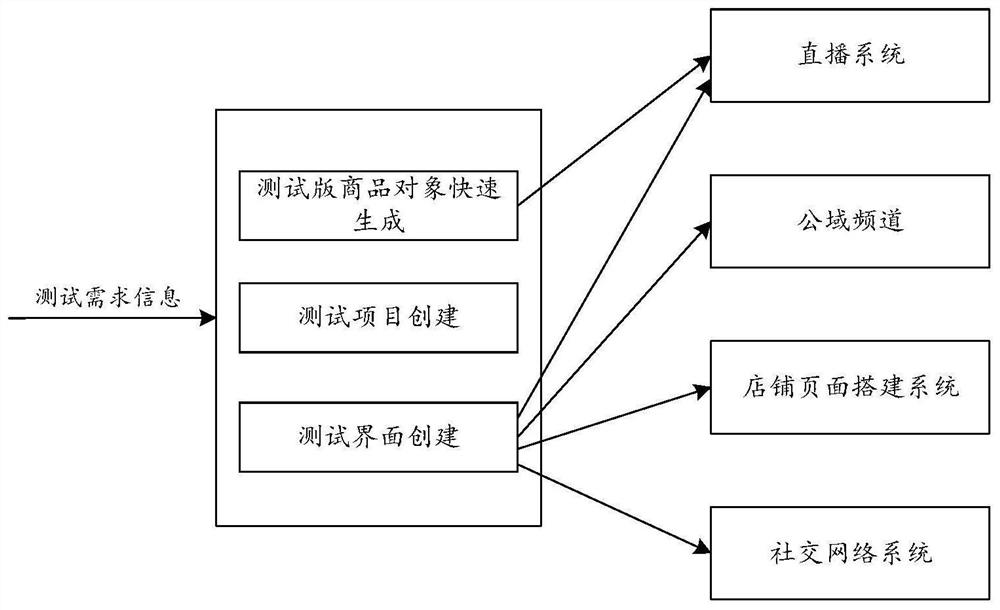
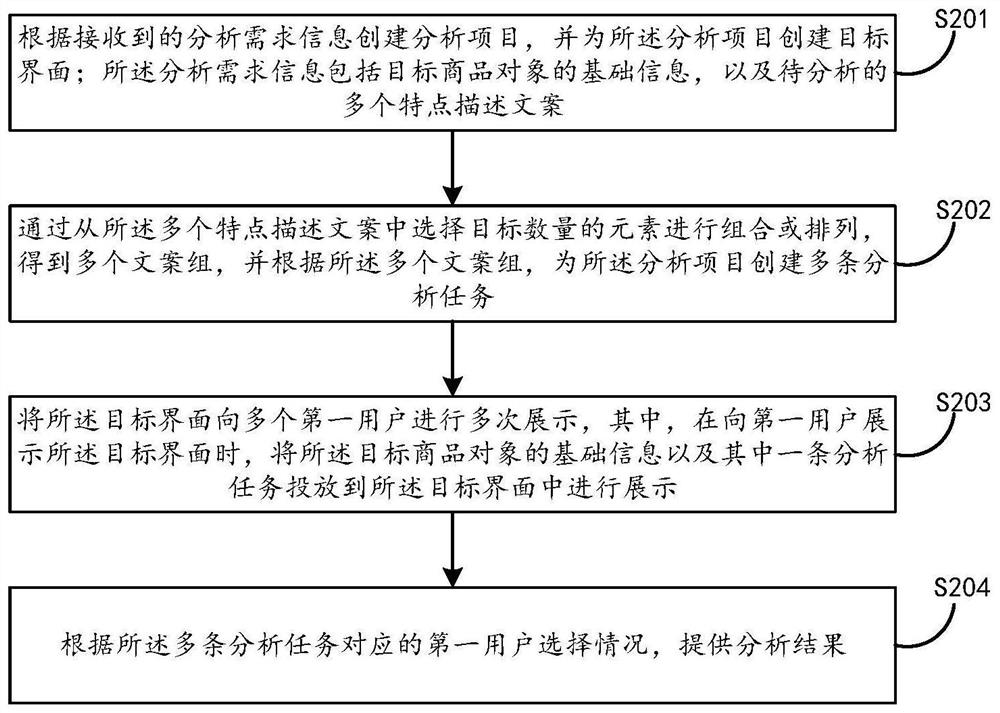
Commodity object analysis method and electronic equipment
PendingCN114187023AGood choiceLower cost of participationMarket predictionsReservationsEngineeringFeature description
The embodiment of the invention discloses a commodity object analysis method and electronic equipment, and the method comprises the steps: creating an analysis item according to received analysis demand information, and creating a target interface for the analysis item; the analysis demand information comprises basic information of a target commodity object and a plurality of to-be-analyzed feature description documents; selecting a target number of elements from the plurality of feature description copywrites for combination or arrangement to obtain a plurality of copywriting groups, and creating a plurality of analysis tasks; displaying the target interface to a plurality of first users for multiple times, and when the target interface is displayed to the first users, putting the basic information of the target commodity object and one analysis task into the target interface for display; and providing an analysis result according to the first user selection condition corresponding to the plurality of analysis tasks. According to the embodiment of the invention, the commodity object can be analyzed more effectively.
Owner:ALIBABA (CHINA) CO LTD
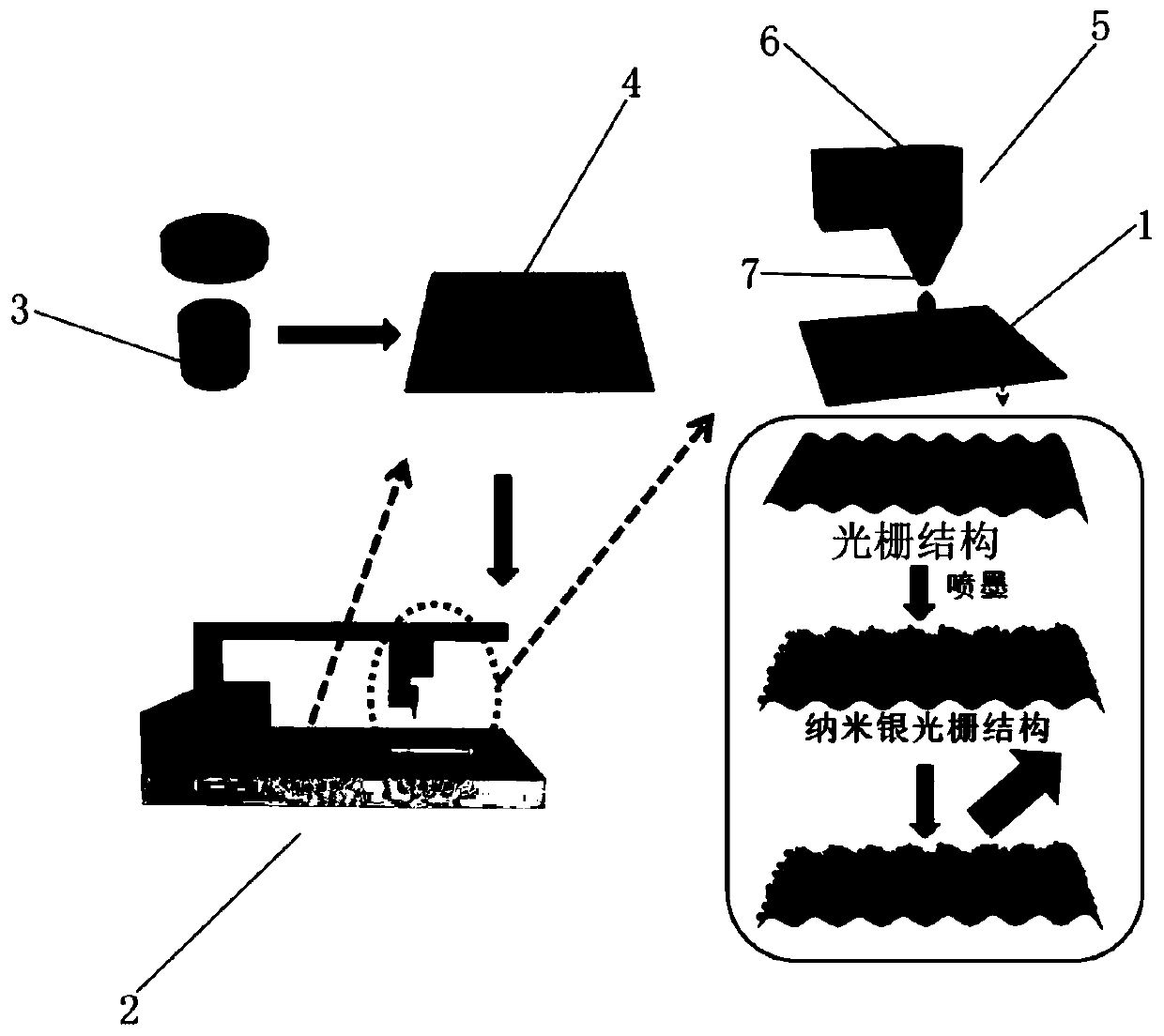
Method for preparing SERS active substrate based on grating structure of optical disc
InactiveCN109765650ALow priceUniform grating structureRaman scatteringDiffraction gratingsGratingEngineering
The invention relates to the field of preparation of SERS active substrates, and discloses a method for preparing an SERS active substrate based on a grating structure of an optical disc. The SERS active substrate is prepared by performing ink jet printing on the grating structure of the optical disc. The method of the invention has the beneficial effects that ink printing can be accurately carried out in a predefined area of the optical disc through an ink jet printing technology, the consumption of ink can be controlled, the ink waste can be reduced, and the SERS substrate with high repetition rate can also be produced.
Owner:SHANGHAI NORMAL UNIVERSITY
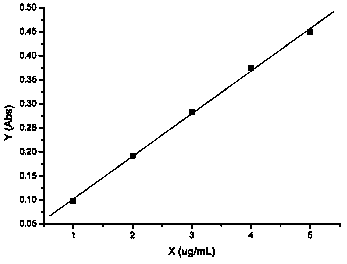
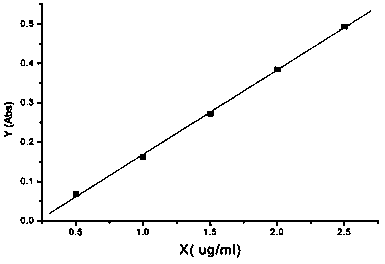
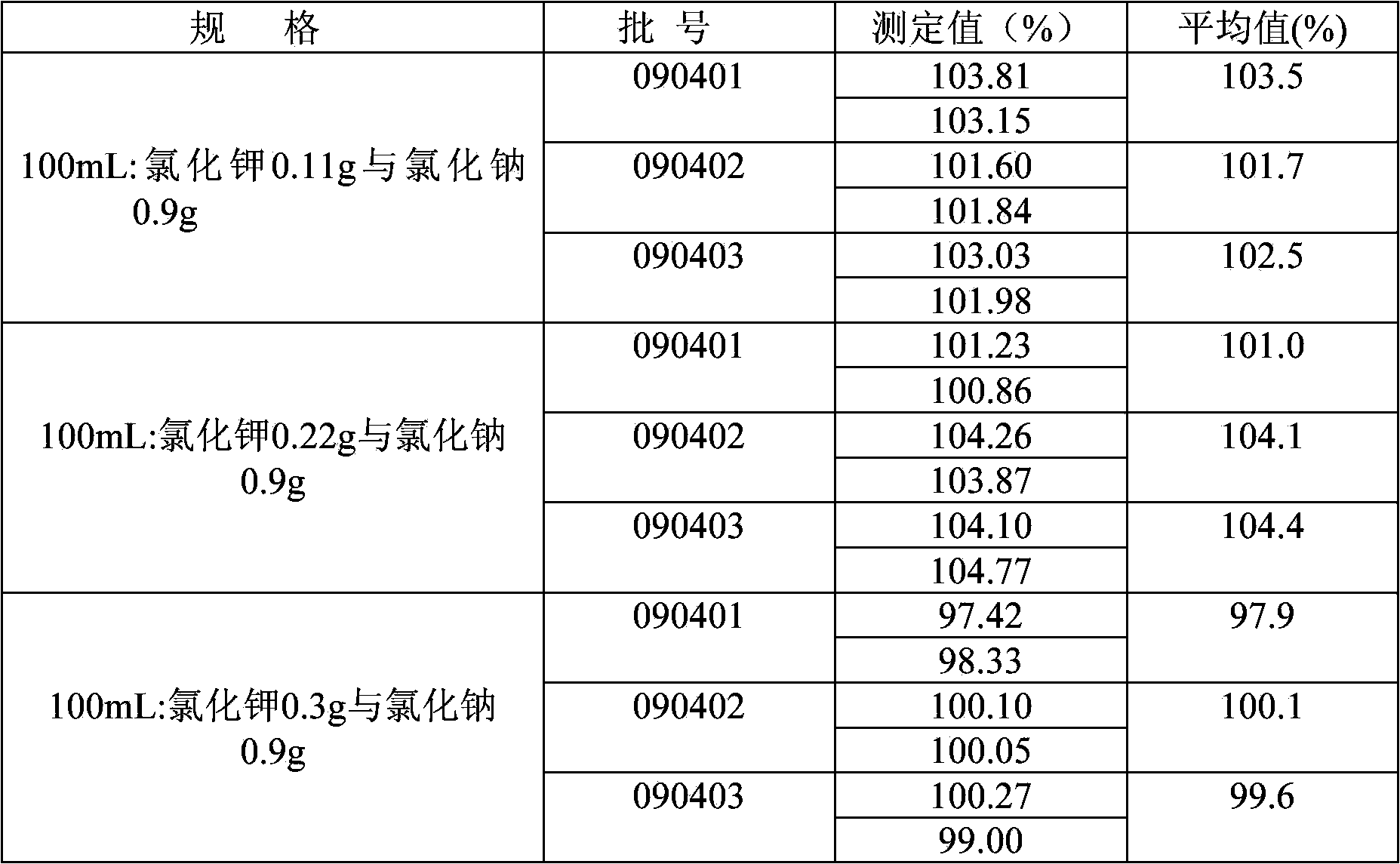
Method for measuring content of potassium chloride and sodium chloride in potassium chloride and sodium chloride injection solution
ActiveCN103575669AEasy to useReduce the chance of secondary pollutionColor/spectral properties measurementsSodium Chloride InjectionPOTASSIUM CHLORIDE/SODIUM CHLORIDE
The invention relates to a method for measuring the content of potassium chloride and sodium chloride in a potassium chloride and sodium chloride injection solution, and a method for determining whether the content of potassium chloride and sodium chloride in the potassium chloride and sodium chloride injection solution meets quality standards. The measuring method is carried out through an atomic absorption spectrometry method by an atomic absorption spectrophotometer.
Owner:SICHUAN QILI PHARM CO LTD
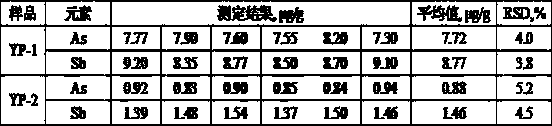
Method for simultaneously and rapidly determining arsenic and antimony in sodium stannate by vapor generation-atomic fluorescence spectrometry
InactiveCN111351776APromote formationReduce usageFluorescence/phosphorescenceAtomic fluorescence spectrometryPhysical chemistry
The invention relates to a method for simultaneously and rapidly determining arsenic and antimony in sodium stannate by a vapor generation-atomic fluorescence spectrometry (VG-AFS). According to the method, a certain amount of citric acid and hydrochloric acid are used for dissolving sodium stannate, a standard addition method is adopted for determination, under the optimal concentration of citricacid and hydrochloric acid, matrix tin is not hydrolyzed, determination of arsenic and antimony by VG-AFS cannot be interfered, and simultaneous and rapid determination of arsenic and antimony in sodium stannate can be achieved. The method is high in sensitivity and accuracy, good in selectivity and low in detection limit, and the lower detection limit of arsenic and antimony is smaller than or equal to 0.00005%; and the method has the advantages of short analysis time, low cost, no matrix interference, safety, environmental protection and high efficiency, and can well satisfy the requirements of simultaneous rapid analysis of impurity elements arsenic and antimony in sodium stannate.
Owner:北矿检测技术股份有限公司 +1
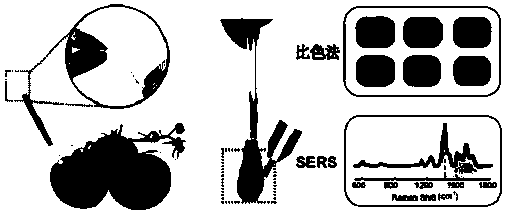
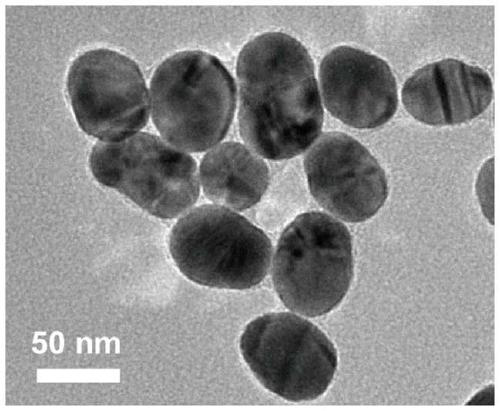
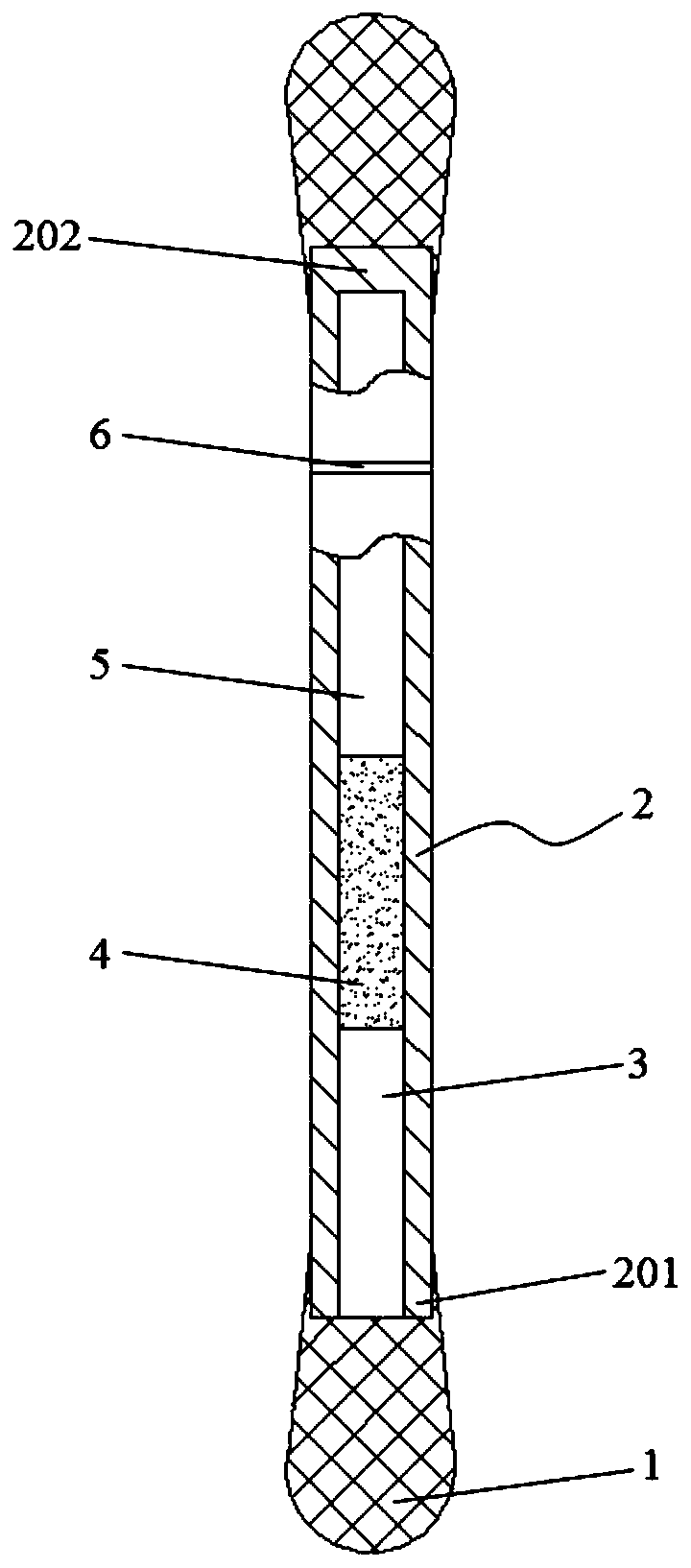
Portable disposable surface-enhanced Raman/colorimetric dual sensor as well as preparation method, detection method and application thereof
ActiveCN111537491AMeet the needs of rapid analysisConvenient disposable disposableMaterial analysis by observing effect on chemical indicatorRaman scatteringPhysicsCitrate sodium
The invention relates to a portable disposable surface-enhanced Raman / colorimetric dual sensor as well as a preparation method, a detection method and application thereof. The sensor comprises a sample measuring tube and a sampling part. The sample measuring tube comprises a tube body, an opening end and a sealing end arranged at two ends of the tube body, a detection solution section arranged inthe tube body, and a closed section. The preparation method comprises the following steps: preparing an ion nanoparticle colloidal solution by adopting a sodium citrate reduction method, and injectingthe ion nanoparticle colloidal solution into the tube body in a vacuum state to obtain the sensor. The detection method comprises the following steps: wiping the surface of a to-be-detected object through a sampling part; breaking the closed section of the tube body to immerse a detection solution into the sampling part; and then performing colorimetric detection or in-situ SERS detection on a discoloration sampling part to obtain qualitative and quantitative analysis results. Compared with the prior art, the portable disposable surface-enhanced Raman / colorimetric dual sensor can meet the requirement for large-batch rapid analysis in a laboratory, is convenient to use at a time in pollution field detection to avoids cross contamination; and a novel portable device for fruit and vegetablepesticide residue detection, emergency analysis and judicial expertise can be provided.
Owner:SHANGHAI INSTITUTE OF TECHNOLOGY
Genetic sample analysis method and electronic equipment
ActiveCN107480468BMeet the needs of rapid analysisSmall footprintDatabase management systemsProteomicsData miningSample selection
Owner:UNITED ELECTRONICS
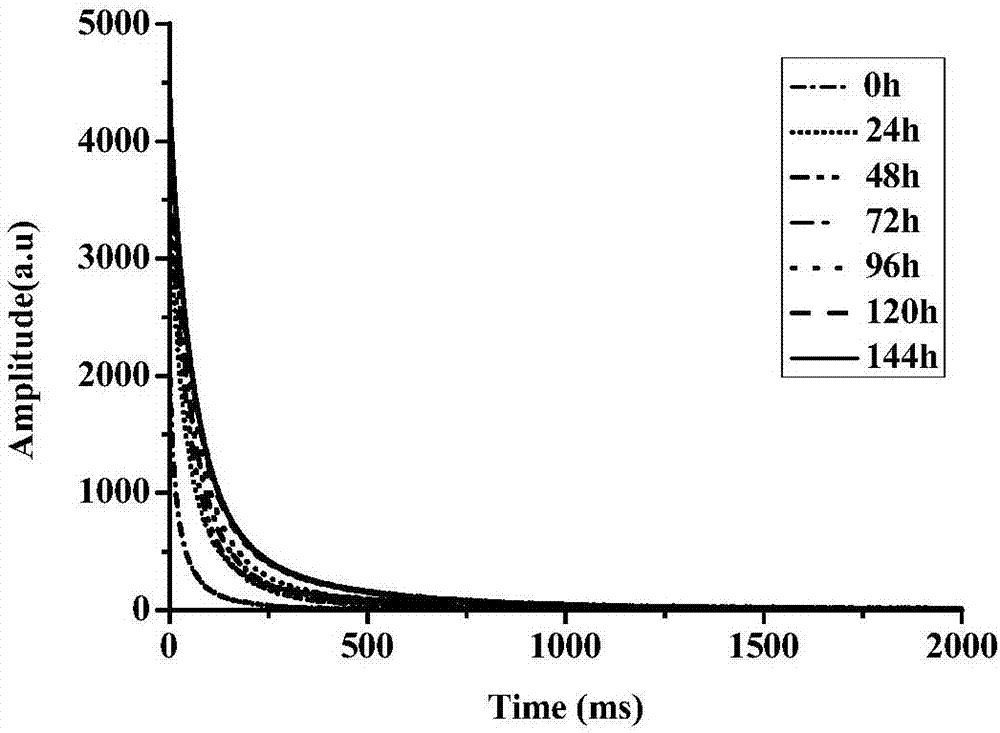
A Method for Detecting Water and Fat Content in Abalone Using Low Field Nuclear Magnetic Resonance Technology
ActiveCN105606637BEasy to operateMeet the needs of rapid analysisAnalysis using nuclear magnetic resonanceLow field nuclear magnetic resonanceMoisture
The invention provides a method for detecting the water content and fat content in abalone through a low-field nuclear magnetic resonance technology. The method comprises the following first step of collecting samples, the second step of carrying out low-field nuclear magnetic analysis on the sample, the third step of measuring the samples, the fourth step of establishing a model, the fifth step of evaluating the model, and the sixth step of measuring the water content and the fat content of the samples to be detected. By means of the method, the water content and fat content in abalone can be measured at the same time, detection is rapid and accurate and free of the influence of the surface feature of abalone, and abalone is not damaged in the measuring process.
Owner:DALIAN POLYTECHNIC UNIVERSITY
A kind of water quality quaternary ammonium salt semi-quantitative detection method
ActiveCN104237219BMeet the needs of rapid analysisEasy to operateMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsWater qualityThymol blue
The invention discloses a semi-quantitative detection method for water quality quaternary ammonium salt, which comprises the following steps: absorbing the water sample to be tested into a reaction tube, adding a quaternary ammonium salt detection reagent dropwise into the reaction tube, and immediately mixing with the detection standard color card after shaking well Colorimetry, compare the color of the detection standard color card to determine the content of the quaternary ammonium salt. The quaternary ammonium salt detection reagent comprises dadan yellow, EDTA, thymol blue and sodium hydroxide, and the component ratio is: dadan yellow: EDTA: thymol blue: sodium hydroxide is 100 by mass ratio: (5-100): (20-200): (10-100) mixed. The detection method of the present invention can quickly detect the concentration of quaternary ammonium salts in water samples only by using quaternary ammonium salt detection reagents, reaction tubes, and detection standard color cards.
Owner:ZHEJIANG UNIV
Method for detecting microcystic toxins in water
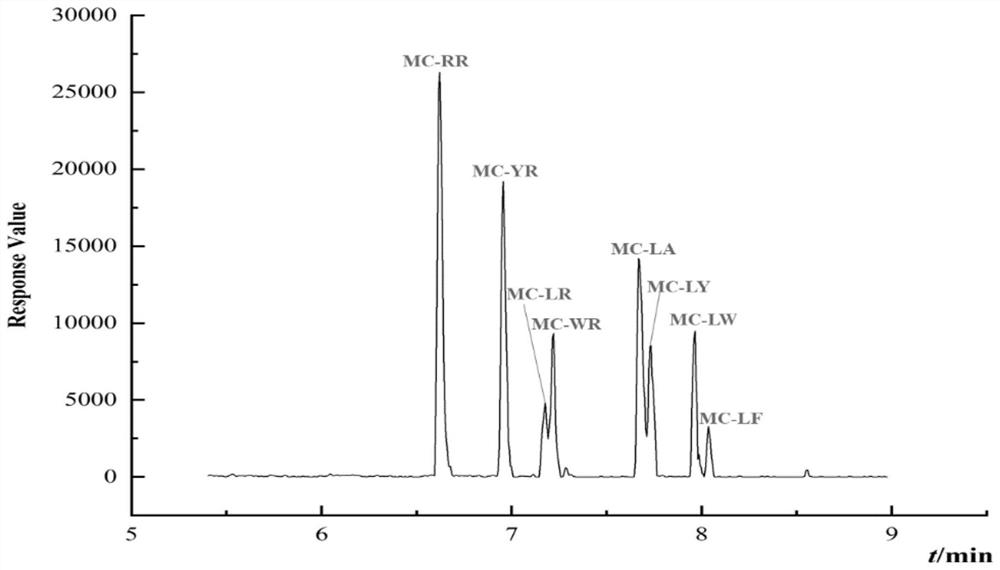
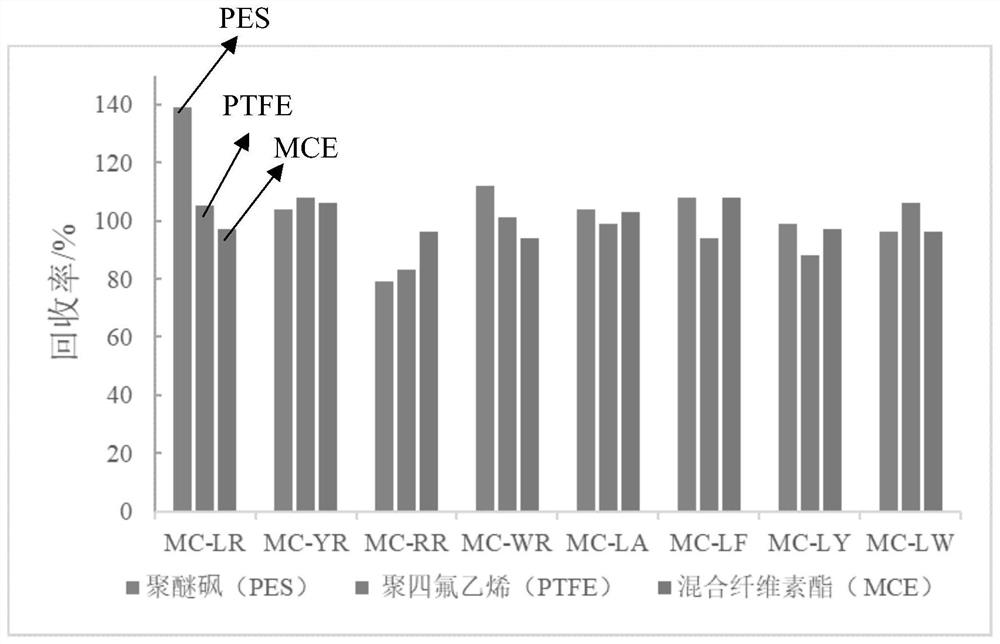
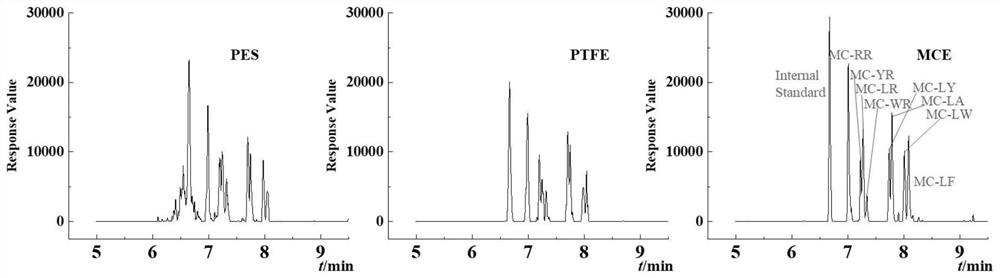
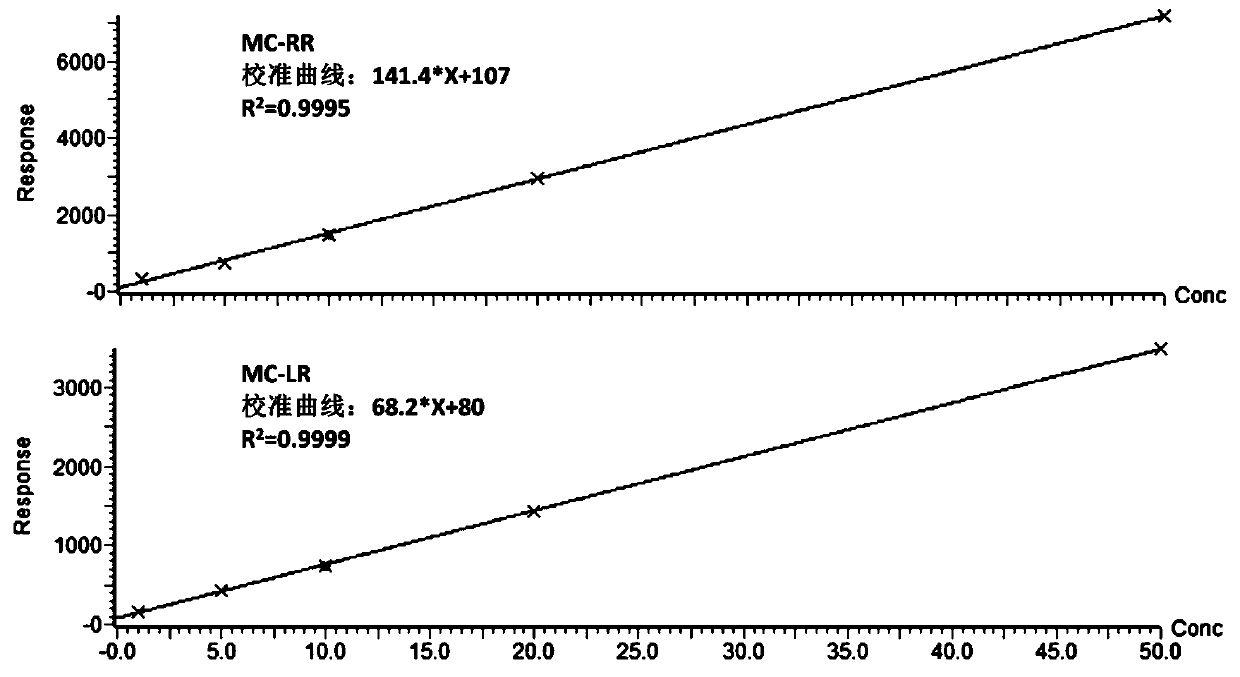
InactiveCN113655147AEfficient detectionCost-effectiveComponent separationColumn temperatureGradient elution
The invention relates to a method for detecting microcystic toxins in water, which comprises the following steps: filtering a water sample through a 0.22 mu m MCE filter membrane, and then carrying out on-line solid-phase extraction and analytical determination on the filtered water sample through an on-line solid-phase extraction ultra-high performance liquid chromatography-tandem mass spectrometry method, wherein the online solid-phase extraction adopts a C8 solid-phase extraction column, the conditions of the ultra-high performance liquid chromatography are as follows: in a mobile phase, a solution A is a 0.1% formic acid acetonitrile solution, and a solution B is a 0.1% formic acid aqueous solution; the gradient elution procedure includes that in 0-4.6 min, and 98% of the solution B is used; in 4.6-10.0 min, 98% liquid B and 25% liquid B are used, and in 10.0-11.0 min, 25% liquid B and 100% liquid B are used; the flow rate is 0.4 mL / min; the column temperature is 35 DEG C, and the sample injection volume is 2mL. The method disclosed by the invention is a method which is high in sensitivity, high in accuracy, good in recovery rate and reproducibility, simple, convenient and rapid to operate and capable of simultaneously determining the eight MCs in the water body.
Owner:CHINESE RES ACAD OF ENVIRONMENTAL SCI
Analytic pretreatment machine for metal material and treatment method for analytic pretreatment machine
The invention relates to an analytic pretreatment machine for a metal material, also relates to a corresponding treatment method and belongs to the technical field of metallurgy detecting and grinding equipment. The analytic pretreatment machine comprises a vertical cylindrical vacuum chamber provided with an air exhaust interface and a sealing door; the middle of the bottom surface of the vacuumchamber upwards extends to form a cathode tube connected with a high-voltage power supply; a cathode head with a placing area for a to-be-treated sample is arranged on the top of the cathode tube; a shielding device for covering the cathode head and the cathode tube is extended from the periphery of the placing area; a cooling channel which is externally connected with a cooling fluid source is arranged inside the cathode head; a grounded anode base is arranged at the middle of the top end of the vacuum chamber; the grounded anode base downwards extends to form an anode head opposite to the cathode head; an inert gas feeding pipe is arranged in the anode head; and a gas exhausting port of the gas feeding pipe is opposite to the sample placing area of the cathode head. Due to the adoption of the analytic pretreatment machine and the treatment method disclosed by the invention, secondary oxidation can be avoided; excellent effect and high speed can be obtained; and the demand for quick analysis can be better met, and thereby a more accurate detection result is obtained.
Owner:NANJING HEAO ELECTROMECHANICAL EQUIP
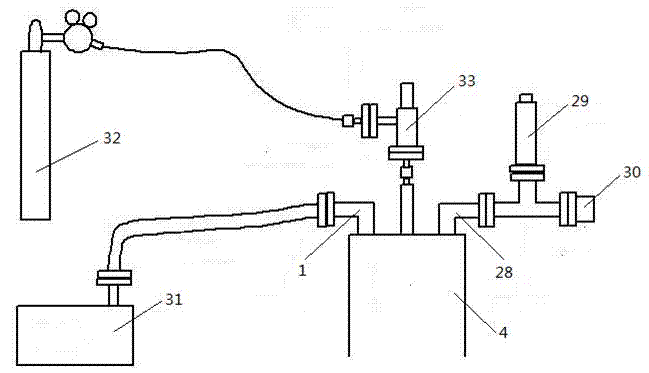
Rapid determination of total iron content in vanadium titanomagnetite
InactiveCN104181272BEasy to operateQuick breakdownMaterial analysis by observing effect on chemical indicatorChemical analysis using titrationMagnetiteSodium diphenylamine sulfonate
The rapid determination method of total iron content in a kind of vanadium-titanium magnetite provided by the invention comprises: 1) taking by weighing the vanadium-titanium magnetite sample whose weight is M; 2) adding a sufficient amount of ore dissolving solution to be measured Vanadium-titanium-magnetite sample is heated and dissolved; 3) Add dilute hydrochloric acid in the reaction vessel; 4) Then dropwise add tin protochloride solution to light yellow, heat to slightly boiling again, then cool to room temperature; 5) Add to the reaction vessel Add sodium tungstate solution to the container, drop titanium trichloride solution to blue, and add dropwise titanium trichloride solution to a slight excess; 6) then drop potassium dichromate solution to colorless, and add diphenylamine sulfonate dropwise sodium acid indicator; 7) titrate to purple with potassium dichromate standard titration solution; 8) measure the weight percentage of vanadium in the solution after titration to purple; 9) calculate the steps. The method is simple in operation, fast in determination speed, and the test results are comparable to the national standard method, which can better meet the needs of on-site rapid analysis.
Owner:武汉钢铁有限公司
A rapid and non-destructive detection method for moisture content in abalone drying and rehydration process
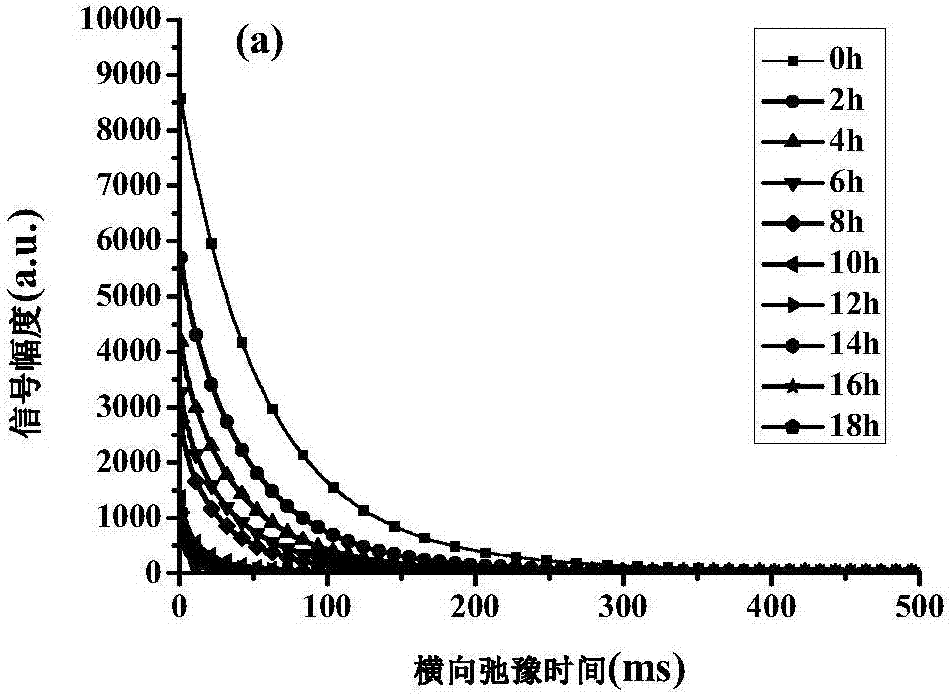
ActiveCN105954309BFast NDTMeet the needs of rapid analysisAnalysis using nuclear magnetic resonanceNon destructiveMoisture
The invention relates to a method for rapid and nondestructive detection of the water content in the abalone drying and rewatering process. The method comprises the following steps: acquiring echo attenuation curve data of fresh abalone samples, drying the fresh abalone samples, and acquiring the CPMG signals of the fresh abalone samples in the drying process; rewatering the dried abalone samples after the echo attenuation curve data of the fresh abalone samples is acquired, and acquiring the CPMG signals of the dried abalone samples in the rewatering process; determining the real water content of every sample; establishing a water content predicating model corresponding to the real water content in the drying and rewatering process; and acquiring the echo attenuation curve data of an abalone sample to be detected through adopting the same technology, and combining with the water content predicating model to obtain the predicated value of the water content of the abalone sample to be detected. The method has the advantages of high measurement result accuracy, short time, no damages to abalones, fastness, and no pretreatment of the abalones, and can realize rapid and nondestructive detection of the water content of the abalone drying and rewatering process.
Owner:DALIAN POLYTECHNIC UNIVERSITY
An Analytical Method for Limiting Short-Circuit Current Using Line Out-of-Series Operation
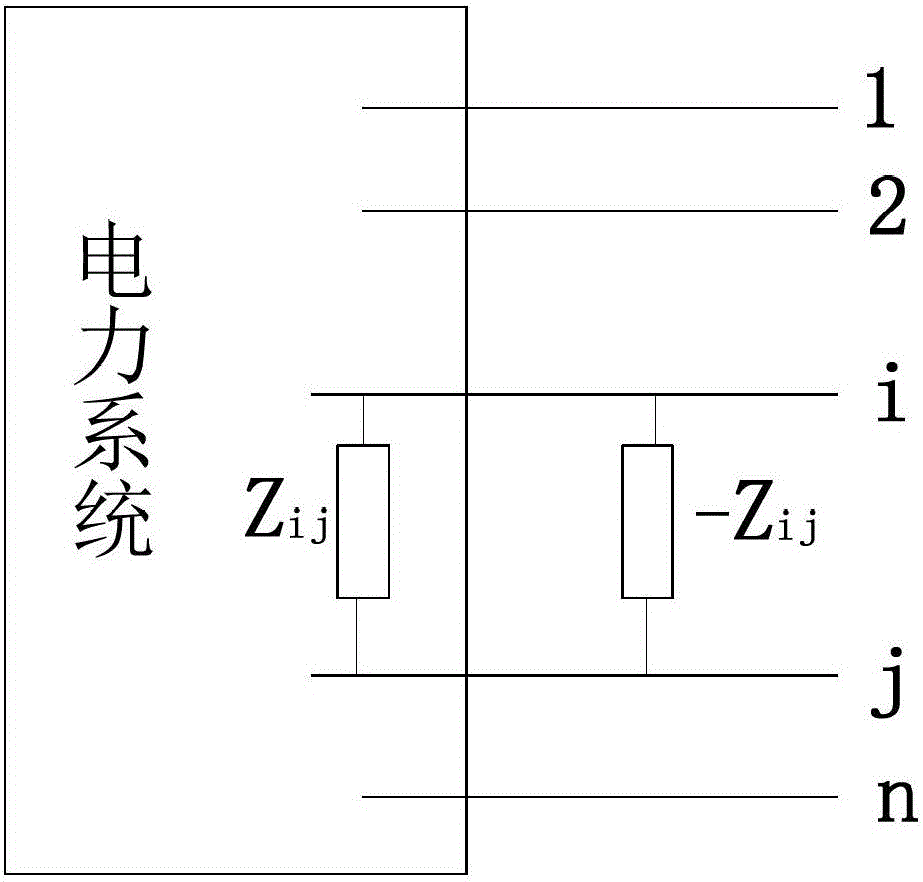
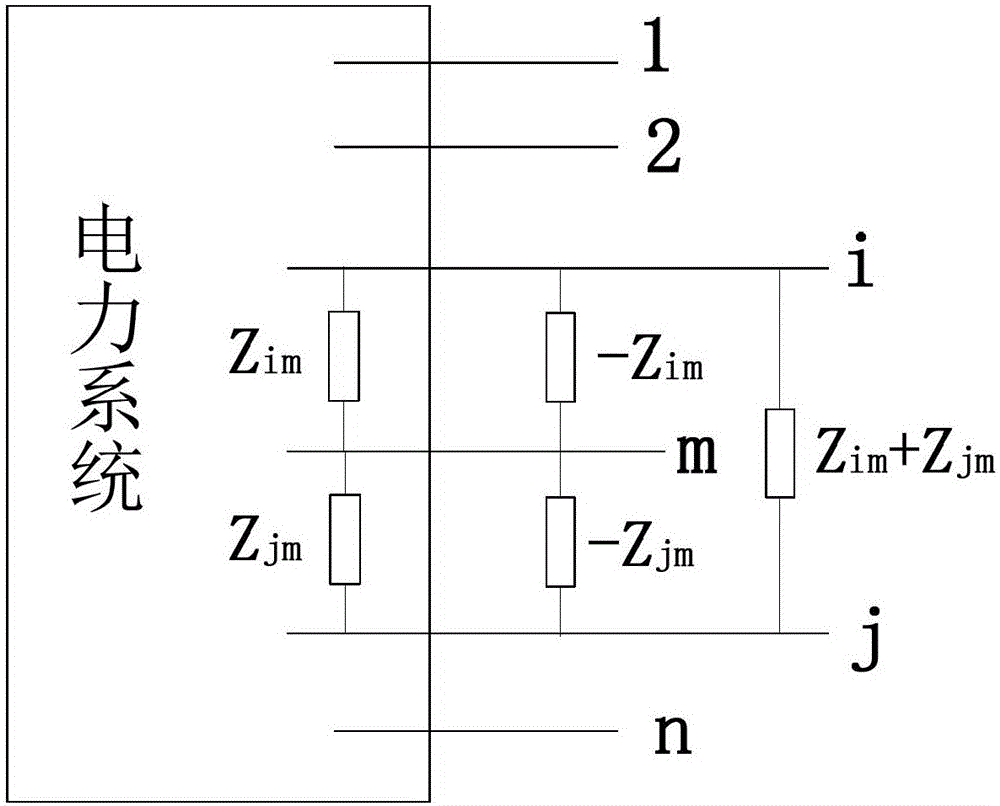
ActiveCN103219719BMeet the needs of rapid analysisCalculation speedEmergency protective arrangements for limiting excess voltage/currentCurrent limitingElectric power system
The invention relates to an analytical method of limiting short-circuit current by utilizing circuit serial operation. The analytical method of limiting the short-circuit current by utilizing circuit serial operation comprises the following steps: (1) an impedance matrix and node short-circuit current of a power grid in a initialization operation mode are calculated; (2) an equivalent circuit corresponding to the circuit serial operation is constructed, and node impedance variable quantities in different circuit serial operations are rapidly calculated; and (3) a serial operation mode when impedance variation is the largest is selected, and the corresponding short-circuit current is calculated. The technical scheme of the analytical method of limiting short-circuit current by utilizing circuit serial operation is applied to analytical calculation of the short-circuit current in an electrical power system and rapid analysis and decision of a limiting scheme of the short-circuit current. The different circuit serial operation modes in the actual operation of the power grid are different in limiting effects on the short-circuit current, the short-circuit current is effectively limited by selecting an appropriate circuit serial operation mode, assistance is provided for the operation mode of the power grid, and the short-circuit current of the power grid is guaranteed not to be out of limits.
Owner:STATE GRID CORP OF CHINA +1
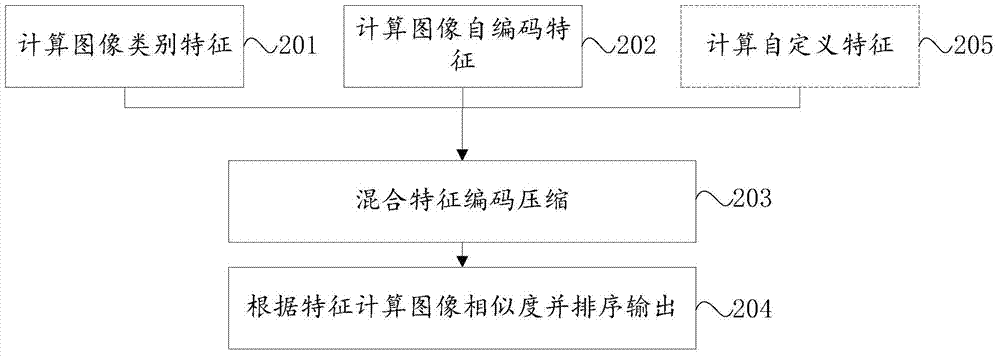
A method and system for image search based on deep learning
ActiveCN104679863BReduce dimensionalityFast search resultsBiological neural network modelsSpecial data processing applicationsFeature extractionClassification methods
The present invention relates to the technical field of image search, and provides a method for image search based on deep learning, in which image category features are calculated, and a trained deep convolutional neural network is used to extract classification features from input images; Encoding features, using the trained deep learning automatic encoding algorithm, extracting encoding features for the input image; mixing feature encoding compression, integrating the classification features and image self-encoding features, and encoding these features through the deep learning automatic encoding algorithm; according to The feature computes image similarity and ranks the output. The present invention utilizes a deep convolutional neural network to generate high-level features to ensure similarity in the image category of image search results; and uses an automatic encoding algorithm to generate low-level image encoding features to ensure that images are similar in content; hybrid self-encoding features The method further integrates classification features and image self-encoding features to reduce the dimensionality and make the search results faster and more stable.
Owner:武汉众智数字技术有限公司
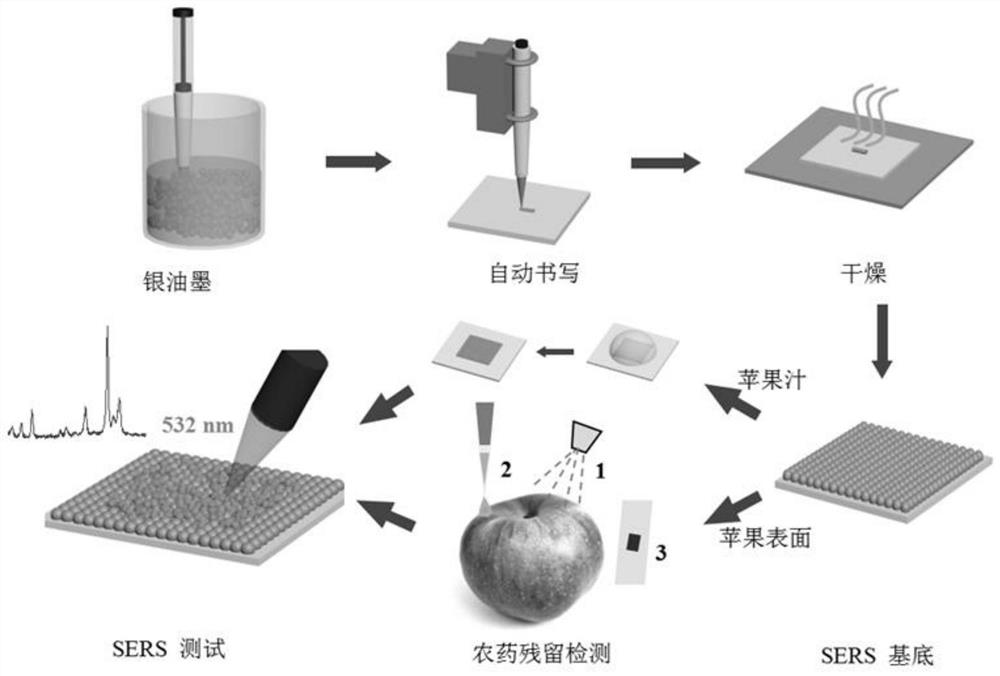
Preparation method and application of automatic writing type SERS substrate
The invention relates to a preparation method of an automatic writing type SERS substrate. The method comprises the following steps: carrying out ultrasonic treatment on metal nanoparticle printing ink, uniformly mixing, and putting into a pen; fixing a paper substrate and the pen to an automatic writing operation platform, and setting parameters of the automatic writing operation platform, wherein the parameters comprise the writing pattern shape, the writing speed and the distance between the pen point and the paper substrate; writing; drying the paper substrate with the metal nanoparticles attached to the surface to obtain an automatic writing type SERS substrate; and cutting the obtained SERS substrate, sealing and storing at normal temperature for later use, wherein the paper substrate is a bendable hydrophobic paper substrate, and the paper substrate does not need to be pretreated. The invention also relates to the automatic writing type SERS substrate prepared by the method. The automatic writing type SERS substrate has the advantages of simple and rapid preparation, low cost, high sensitivity, uniformity, stability and repeatability, and is suitable for trace detection of various substances.
Owner:SHANGHAI NORMAL UNIVERSITY
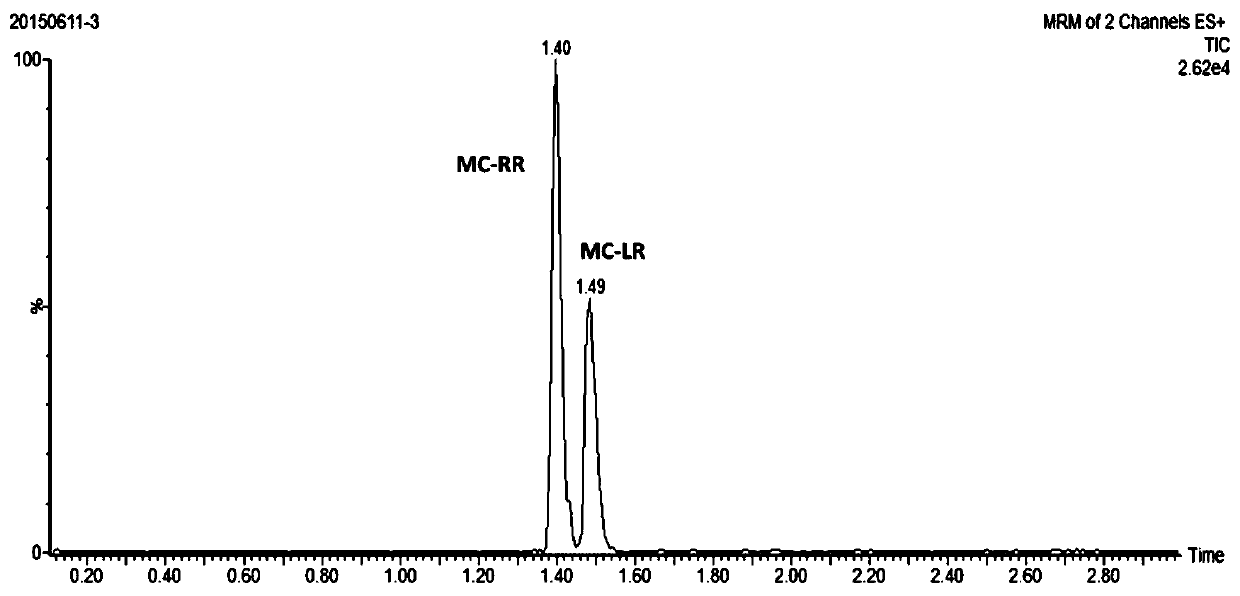
A method for rapid detection of microcystin in water
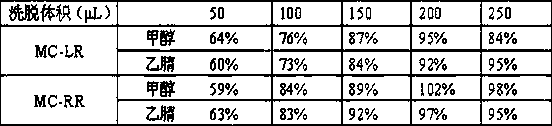
ActiveCN108426971BReduce distractionsHigh sensitivityComponent separationRelative standard deviationTreated water sample
The invention discloses a method for quickly detecting microcystins in water. The method comprises the following steps: 1, filtering an obtained water sample, enriching and purifying the water samplewith a transition metal sulfide nano-material, and quickly passing the water through a filter, adjusting pH of the water sample to 1-5, and finally performing elution with pure water and methanol; 2,analyzing and determining the treated water sample with ultra-high performance liquid chromatography-tandem mass spectrometry. After the water sample is enriched and purified by the transition metal sulfide nanomaterial, MC-LR and MC-RR sensitivity can reach 20 ng / L and 10 ng / L respectively through cooperation with the ultra-high performance liquid chromatography- tandem mass spectrometry, the whole process time is shorter than 20 min, and dual requirements of the environmental monitoring department for timeliness and accuracy of microcystin detection can be fully met. Standard addition experiments prove that the recovery range of drinking water samples with the method reaches 97%-105%, and the recovery rate for waste water samples reaches 83%-99%, and the relative standard deviation is smaller than 5%. The method has the characteristics of high sensitivity, high analysis speed, high selectivity, simple operation and the like.
Owner:浙江中一检测研究院股份有限公司
A method for determining volatile organic compounds in smoking materials
ActiveCN110763794BReduce processingRealize detectionComponent separationMass Spectrometry-Mass SpectrometryBiology
Owner:CHINA TOBACCO YUNNAN IND
Two-dimensional liquid chromatography-mass spectrometry method for mass analysis of low-molecular-weight heparin
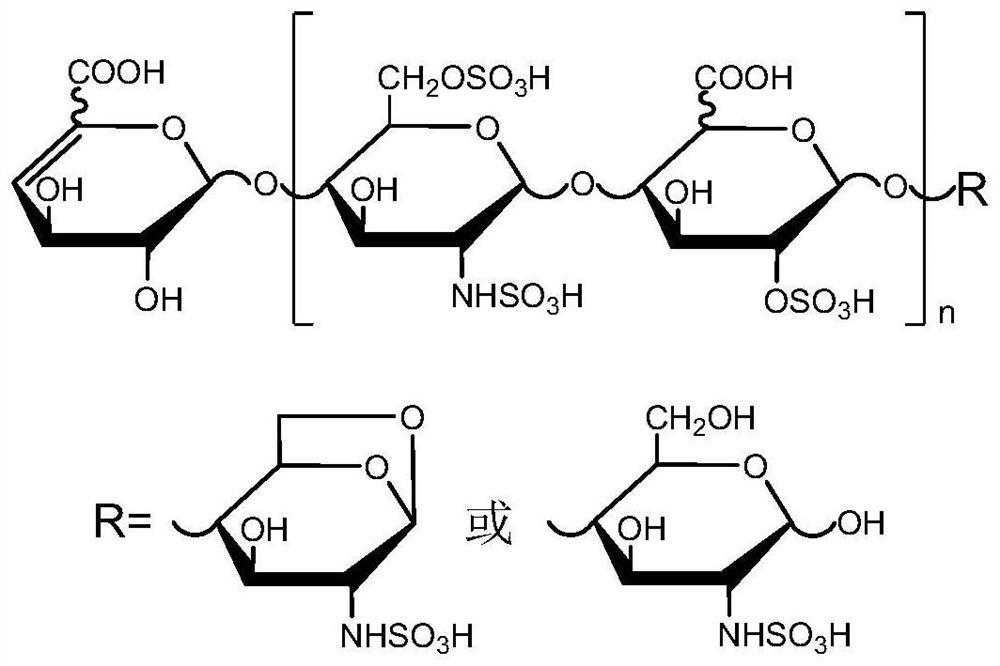
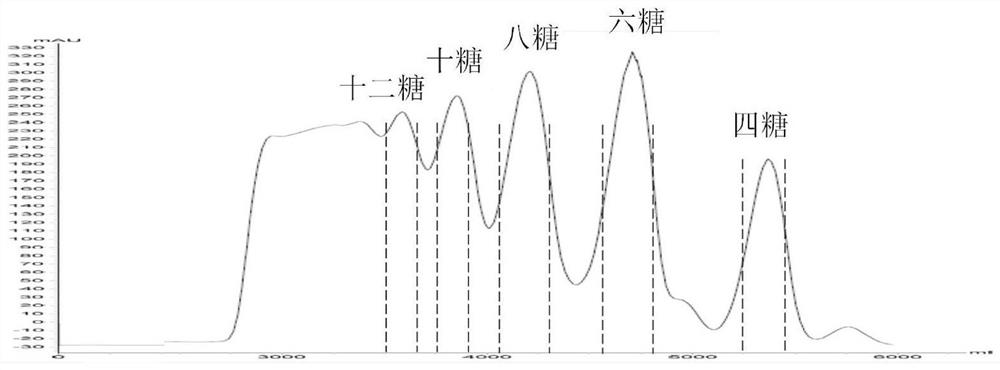
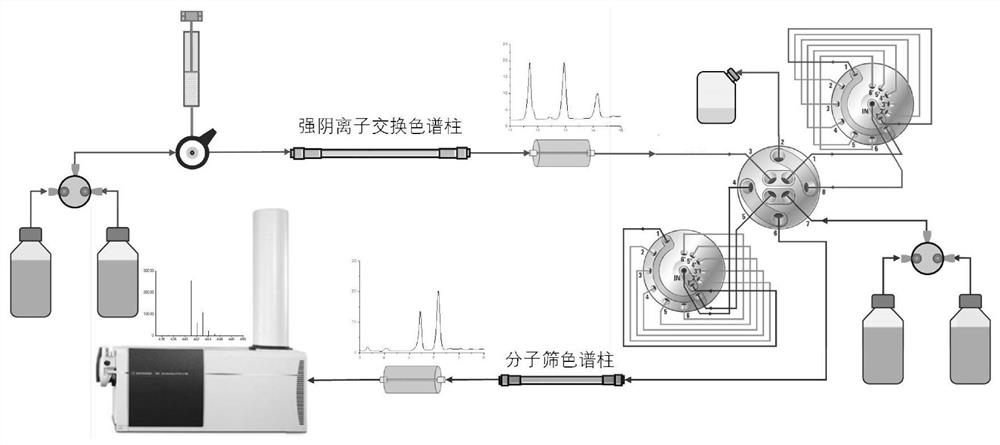
PendingCN114814010AStrong separation abilityMeet the needs of rapid analysisComponent separationHeparin.low molecular weightMolecular sieve
The invention discloses a two-dimensional liquid chromatography-mass spectrometry method for mass analysis of low-molecular-weight heparin, which comprises the following steps: 1) preparing and grading low-molecular-weight heparin as a raw material through medium-pressure column chromatography to obtain a plurality of low-molecular-weight heparin oligosaccharides with different polymerization degrees, and 2) qualitatively analyzing the low-molecular-weight heparin oligosaccharide by using a two-dimensional liquid phase on-line mass spectrometry, namely separating the low-molecular-weight heparin oligosaccharide with a single polymerization degree by using strong anion exchange chromatography in a first-dimensional liquid phase, cutting each SAX separation oligosaccharide peak into a second-dimensional molecular sieve chromatography, and finally, carrying out on-line mass spectrometry. And 3) comparing SAX standard maps of different samples under the same polymerization degree, and carrying out semi-quantitative analysis on oligosaccharides of each polymerization degree through peak areas. The method can be used for quickly and finely analyzing the structure and controlling the quality of the low-molecular-weight heparin, and has huge application potential.
Owner:SUZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com