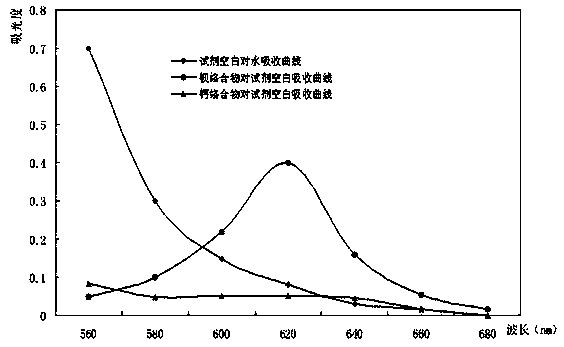
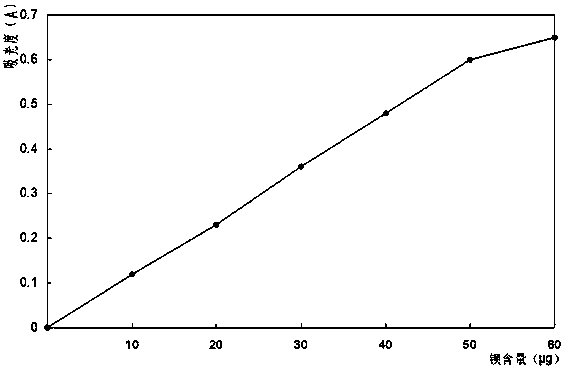
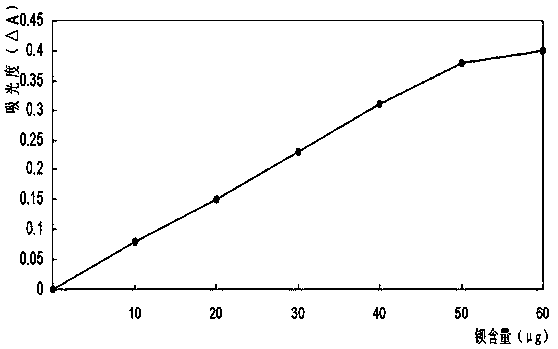
Method for quickly determining content of barium in nickel salt solution
A technology for rapid determination of barium content, applied in measuring devices, color/spectral characteristic measurement, material analysis through optical means, etc., can solve the problems of expensive equipment, small allowable amount of matrix elements, narrow linear range, etc., and achieve stable method Reliable, low co-precipitation effect, high tolerance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Preparation of barium standard solution: Weigh 0.2874g spectrally pure barium carbonate which was pre-dried in an oven at 100°C to constant weight in a 400mL beaker, add 5mL of hydrochloric acid solution, the hydrochloric acid solution is high-grade pure concentrated hydrochloric acid and add it to the same Mix the solution in pure water of 1 volume, heat to dissolve, the heating temperature is 180°C, then cool to room temperature, transfer it into a 200mL volumetric flask, use distilled water to make up the volume, and mix well to obtain a barium standard with a barium ion concentration of 1mg / mL Solution: pipette 5.00mL of the above barium standard solution into a 500mL volumetric flask, dilute to volume with distilled water, and mix well to obtain a solution with a barium ion concentration of 10 μg / mL for use.
Embodiment 2
[0024] Preparation of barium standard solution: Weigh 0.2874g spectrally pure barium carbonate which was pre-dried in an oven at 100°C to constant weight in a 400mL beaker, add 5mL of hydrochloric acid solution, the hydrochloric acid solution is high-grade pure concentrated hydrochloric acid and add it to the same Mix the solution in pure water of 1 volume, heat to dissolve, the heating temperature is 230°C, then cool to room temperature, transfer it into a 200mL volumetric flask, use distilled water to make up the volume, and mix well to obtain a barium standard with a barium ion concentration of 1mg / mL Solution: pipette 5.00mL of the above barium standard solution into a 500mL volumetric flask, dilute to volume with distilled water, and mix well to obtain a solution with a barium ion concentration of 10 μg / mL for use.
Embodiment 3
[0026] Sample treatment: Pipette 1.00mL of nickel salt solution into a 400mL beaker, add 80mL of water, heat to boiling at a temperature of 180°C, remove and stir at a stirring speed of 150 rpm, and add 7mL of sodium hydroxide solution dropwise under stirring , so that the solution in the beaker produces a large amount of precipitation, heat the beaker at 180°C for 5 minutes, remove it and let it stand until all the precipitation sinks, filter it into a 200mL volumetric flask, and wash it again with 8mL of hot water Beaker and precipitate 6 times, and finally adjust the pH value of the filtrate to 0.5 with hydrochloric acid solution, cool the volumetric flask to room temperature, make up to volume with water, and mix well for testing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com