Difunctional macrocyclic chelate, conjugate, metal complex and application thereof
A metal complex and bifunctional technology, applied in the field of bifunctional macrocyclic chelates, can solve the problems of destroying the activity of target molecules and the inability to accurately realize the integration of diagnosis and treatment, and achieve good in vivo and in vitro stability and precise targeting Effect of diagnosis or treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
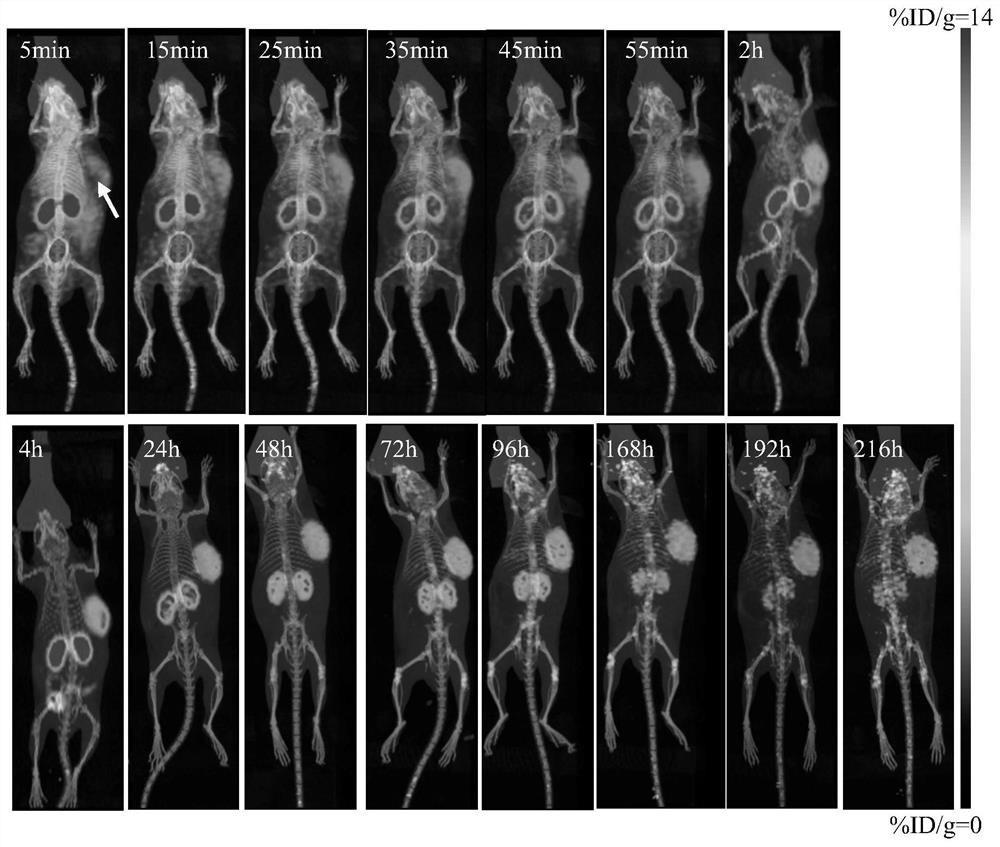
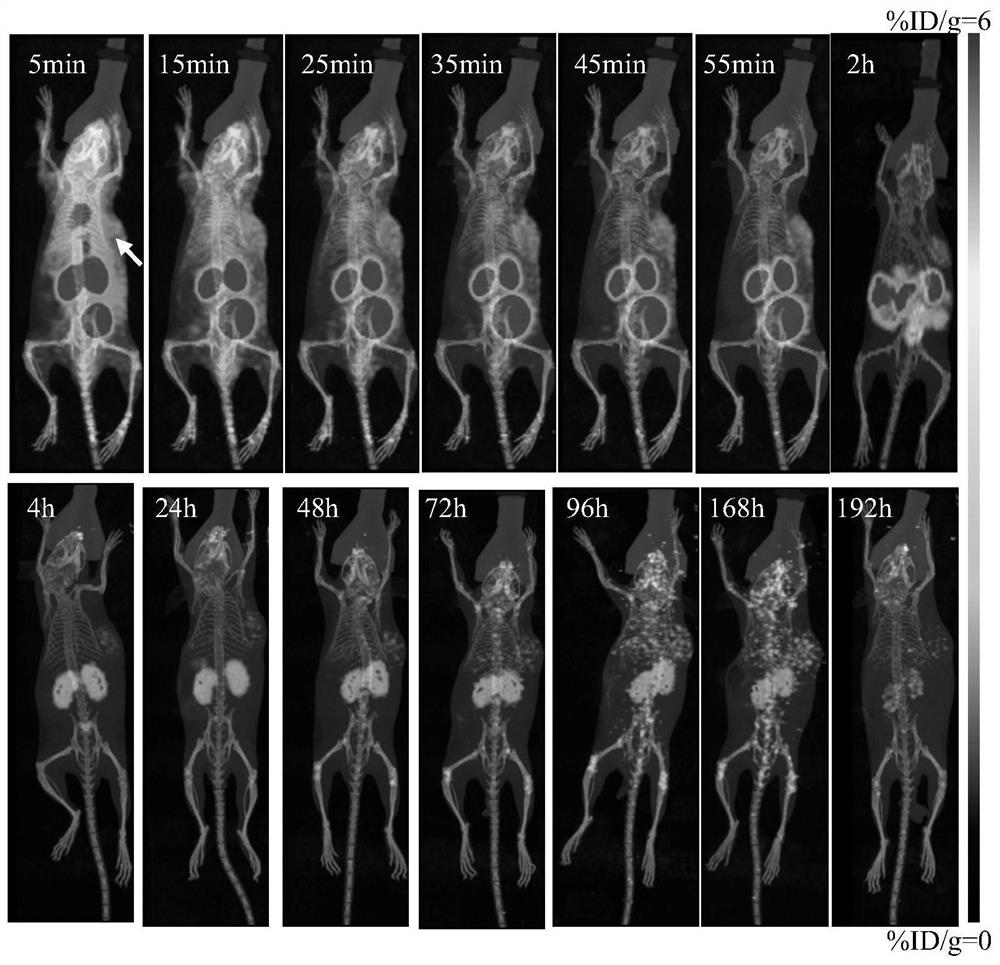
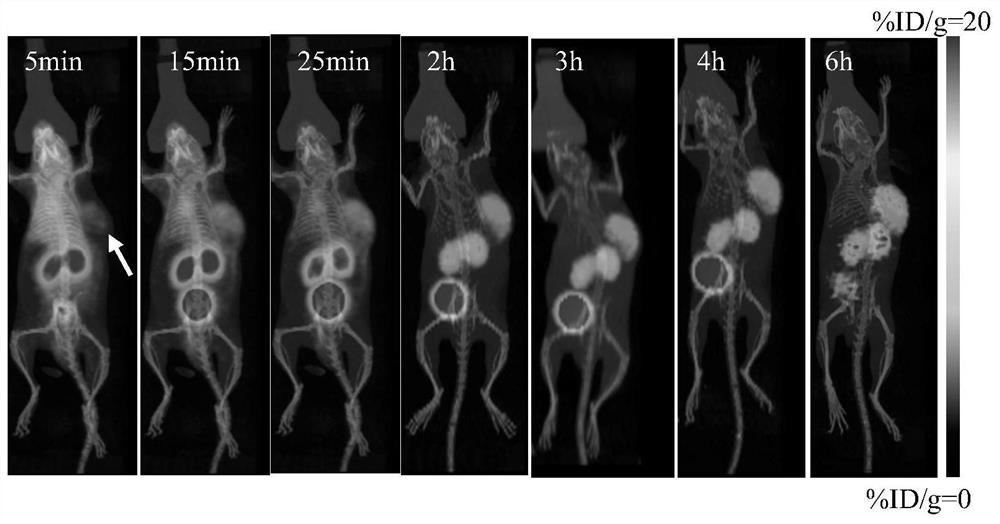
Image
Examples
Embodiment 1
[0056] The present embodiment provides a bifunctional macrocyclic chelate (denoted as Dar), whose structural formula is as follows:
[0057]
[0058] The present embodiment also provides a preparation method of the above-mentioned bifunctional macrocyclic chelate, which is synthesized with reference to the following synthetic route:
[0059]
[0060] The specific operations are as follows:
[0061] step one:
[0062] 2,6-Bis(hydroxymethyl)-4-methylphenol (16.8 g, 0.1 mol) was dissolved in dichloromethane (20 mL). Concentrated hydrochloric acid (100 mL) was added and stirred at room temperature for 24 h. The reaction mixture was concentrated to dryness to give compound A.
[0063] Step 2:
[0064]A solution of urotropine (18.9 g, 0.14 mol) in methanol (380 mL) was added rapidly to the freshly dissolved Compound A (0.061 mol) under vigorous stirring at room temperature. After a few seconds, a fine microcrystalline colorless solid was isolated. The mixture was left at...
Embodiment 2
[0079] The present embodiment provides a bifunctional macrocyclic chelate, and its structural formula is as follows:
[0080]
[0081] The present embodiment also provides a method for preparing the above-mentioned bifunctional macrocyclic chelate, which is synthesized with reference to the following synthetic route:
[0082]
[0083] The specific operations are as follows:
[0084] step one:
[0085] 6,6'-Dimethyl-2,2'-bipyridine (22.1g), NBS (44.7g), benzoyl peroxide (250mg) were added to 1200mL carbon tetrachloride solution, refluxed for 6h, hot filtered, The filtrate was cooled to 0-10°C for insulation, suction filtered, the obtained solid was added to 100 mL of methanol to make a slurry, suction filtered, and the solid was dried to obtain 6.91 g of compound 1.
[0086] LC-MS: 340.9 [M+H] + .
[0087] Step 2:
[0088] Compound 1 (4.25 g) was dissolved in 160 mL of chloroform, heated and refluxed to dissolve, 3.8 g of hexamethylenetetramine was dissolved in 100 m...
Embodiment 3
[0101] The present embodiment provides a bifunctional macrocyclic chelate (denoted as Dar-PEG) whose structural formula is as follows:
[0102]
[0103] The bifunctional macrocyclic chelate in this example is prepared from the bifunctional macrocyclic chelate Dar (1.1eq) of Example 1 and compound G (1eq) through Fmoc solid-phase synthesis reaction, and the condensing agent used is HOAt (2.2eq) And DIC (2.2eq), and then obtained by deprotection. Wherein, the structural formula of compound G is as follows:
[0104] LC-MS: 509.9 [M / 2+H] + , 1018.7[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com