Detection method of p-methoxypropiophenone related substances
A technology of p-methoxybenzene and p-methoxybenzaldehyde, which is applied in the field of chemical analysis, can solve problems such as lack of effective detection, and achieve the effect of improving product quality, high practical value, and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1.1 Preparation of solution:
[0042] Blank solvent: methanol.
[0043] Preparation of mixed reference stock solution: take p-methoxybenzaldehyde, methyl 2-chloropropionate, 4-methoxy-3-methylpropiophenone, 2-methoxypropiophenone, 3-methoxypropiophenone Appropriate amount of Propiophenone reference substance, accurately weighed, quantitatively diluted with methanol to prepare approximately p-methoxybenzaldehyde, 2-chloropropionate methyl ester, 4-methoxy-3-methyl propiophenone, 2 -Methoxypropiophenone, 3-methoxypropiophenone solution of 200 μg each, as a mixed reference stock solution.
[0044] Mixed reference substance solution: Take the mixed reference substance stock solution prepared above, and quantitatively dilute it with methanol to prepare approximately p-methoxybenzaldehyde, methyl 2-chloropropionate, 4-methoxy-3-methyl-4-methoxybenzaldehyde in 1 mL. Propiophenone, 2-methoxypropiophenone, and 3-methoxypropiophenone each had a solution of 20 μg as a mixed refe...
Embodiment 2
[0057] Methodological validation:
[0058] 2.1 Exclusivity
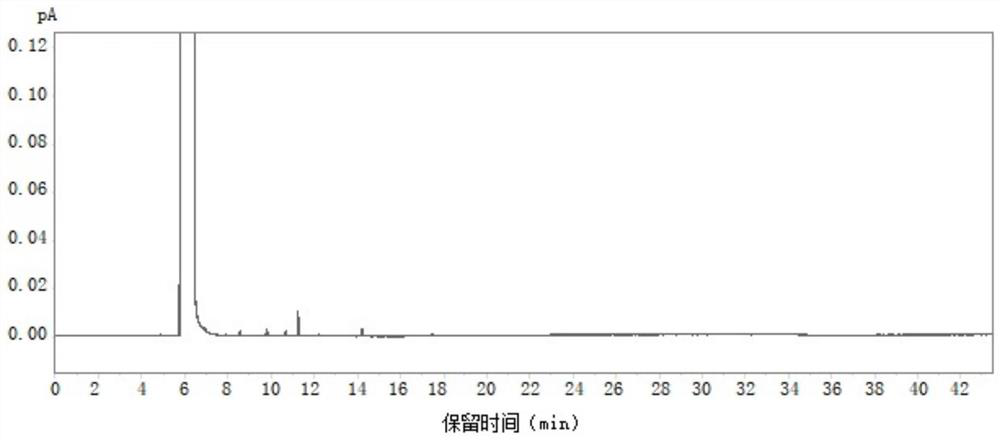
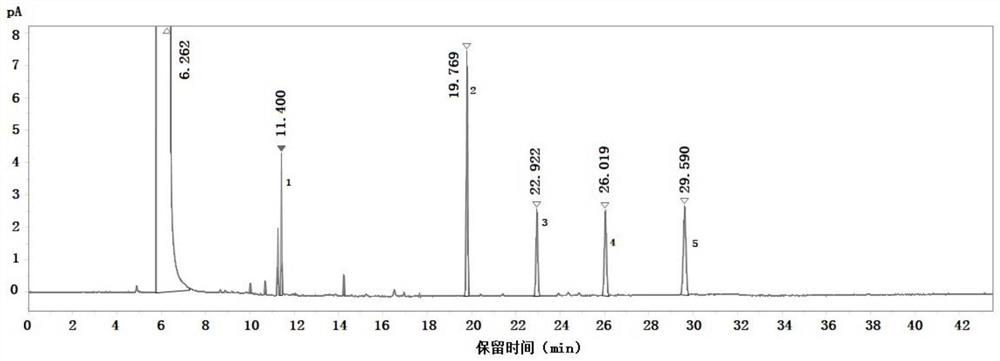
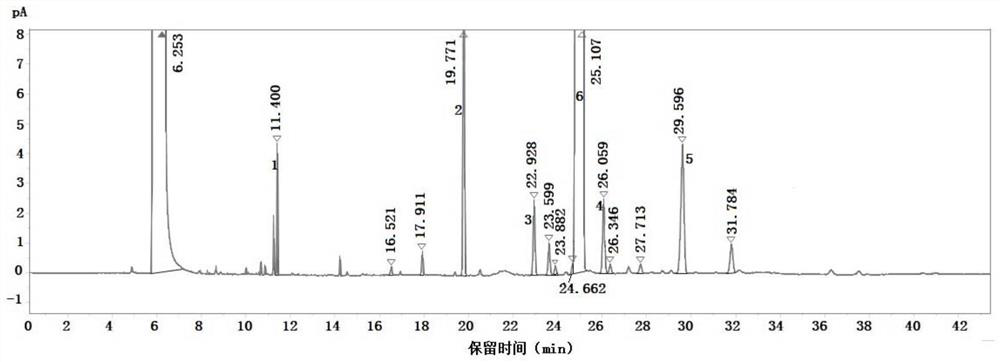
[0059] Take the blank solvent, the above-mentioned mixed reference solution and the standard solution for testing according to the above-mentioned gas chromatographic conditions, and record the chromatogram. The results are as follows: Figure 1-Figure 3 shown, where, figure 1 is the chromatogram of the blank solvent, figure 2 is the chromatogram of the mixed reference solution, image 3 For the chromatogram of the spiked test solution. The test results of the separation degree of each component are shown in Table 1-Table 2.
[0060] Table 1 Test results of the separation degree of each component in the mixed control solution
[0061] Element degree of separation methanol - Methyl 2-chloropropionate 14.78 p-Methoxybenzaldehyde 115.26 2-Methoxypropiophenone 25.85 3-Methoxypropiophenone 19.77 4-Methoxy-3-methylpropiophenone 18.56
[0062] Table 2 The separation...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com