Detection method of triple negative breast cancer immunotherapy curative effect prediction marker CCL19
A technology for triple-negative breast cancer and immunotherapy, applied in the biological field, can solve the problems of poor prediction effect, low accuracy, and impossibility of popularization of immunotherapy curative effect biomarkers, and achieve accurate prediction of immunotherapy curative effect and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
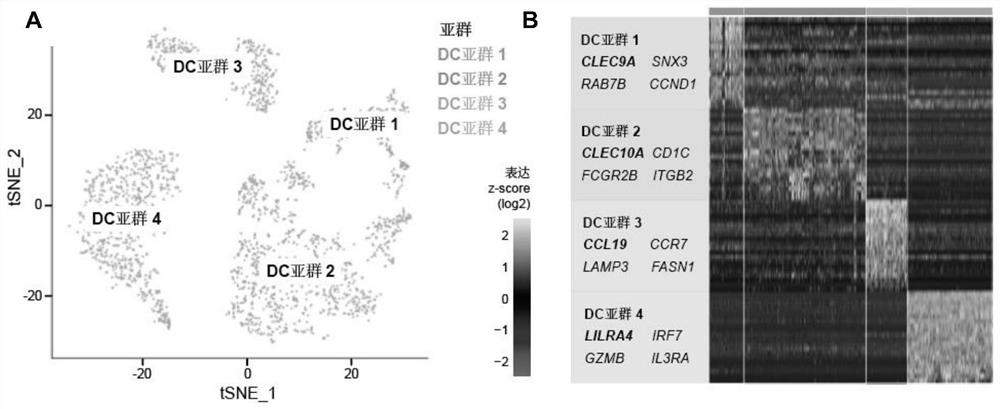
[0048] Example 1 Discovery and functional verification of CCL19 molecules
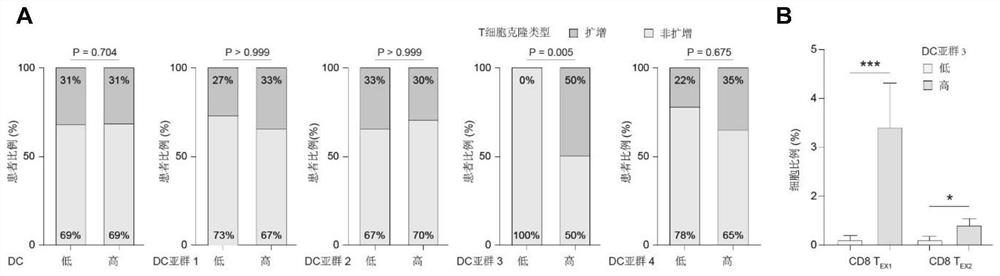
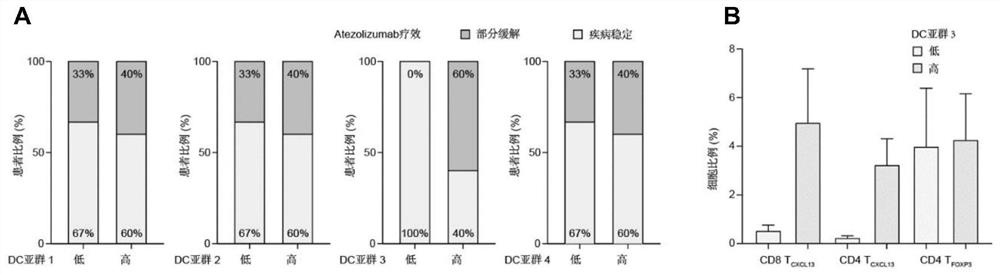
[0049] Dendritic cells (DCs) are the most important antigen-presenting cells, which can initiate T cell-mediated immune responses, but their role in tumor immunotherapy is not yet clear. Based on single-cell sequencing, multi-omics analysis, in vivo experiments and clinical trial validation, the present invention identifies a key DC subset that can predict TNBC immunotherapy response, namely CCL19 + DC (Examples 1.1 and 1.2). After validation by multiple external cohorts, CCL19 + DCs were shown to be widespread in breast cancer tissue, enriched in TNBC and suggest anti-tumor immune activation (Examples 1.3 and 1.4). More critically, we used CCL19 to characterize CCL19 + DC to simplify clinical application, found that CCL19 is mainly composed of CCL19 + DC secretion, and by promoting CD8 + T cells function to exert anti-tumor immunity (Examples 1.5 and 1.6). Therefore, CCL19 molecule can be used a...
Embodiment 2
[0077] Example 2 Serum CCL19 level in peripheral blood of TNBC patients before treatment as a predictive marker of ICI efficacy
[0078] ELISA kit was used to detect the level of CCL19 in the serum of patients with metastatic TNBC before receiving immunotherapy, and it was found that the expression level of serum CCL19 before treatment could predict the benefit of immunotherapy. The specific verification process includes the following steps:
[0079] 1) Study cohort
[0080] We collected sera from 18 patients with metastatic TNBC treated with camrelizumab (PD-1 monoclonal antibody) in the FUTURE clinical trial (international registration number NCT03805399) before and after treatment, using an ELISA kit (Abecam). , Cat. No. ab100601) detected serum CCL19 levels in patients ( Figure 19 ). The immune response assessment can be divided into clinical remission (the sum of CR and PR) and clinical non-remission (the sum of PD and SD). The serum CCL19 concentration before treatm...
Embodiment 3
[0084] Example 3 Immunohistochemical staining of paraffin sections of tumor tissue from TNBC patients before treatment to detect CCL19 as a predictive marker for ICI efficacy
[0085] Immunohistochemistry was used to detect the level of CCL19 in the tissue of patients with metastatic TNBC before receiving immunotherapy, and it was found that the expression level of CCL19 in the tumor tissue before treatment could predict the benefit of immunotherapy.
[0086] We collected pre-treatment paraffin sections from 30 camrelizumab-based patients with advanced TNBC in the FUTURE-C-Plus clinical trial (international registration number NCT04129996), using CCL19 antibody (Sigma, Cat. No. HPA067758, diluted Multiples of 1:200), immunohistochemical staining (the experimental platform was Ventana Benchmark ULTRA detection system), CCL19 positive cells / total cells ≥ 1% were defined as CCL19 positive, and the relationship between it and the efficacy of patients was evaluated. Among the 29 pa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com