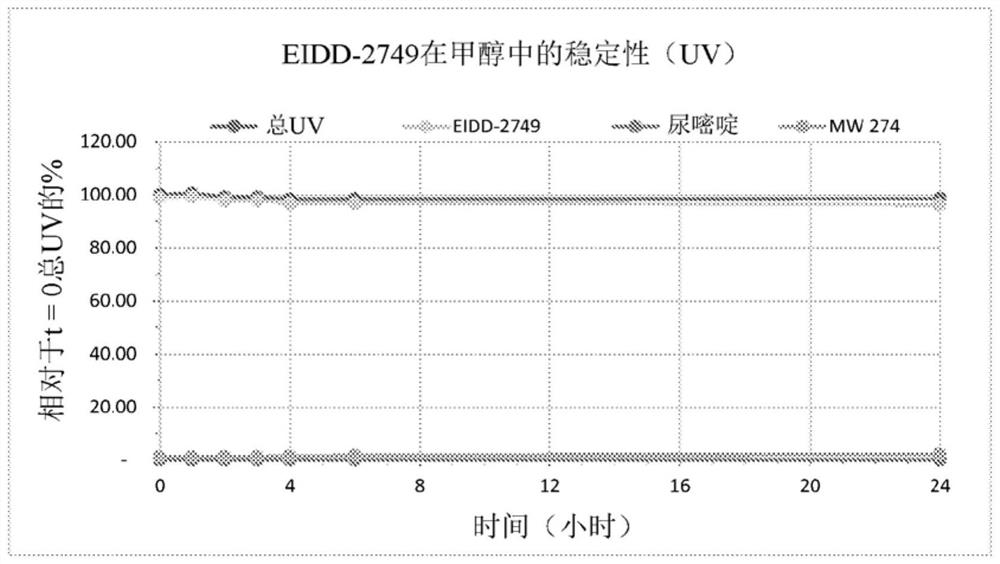
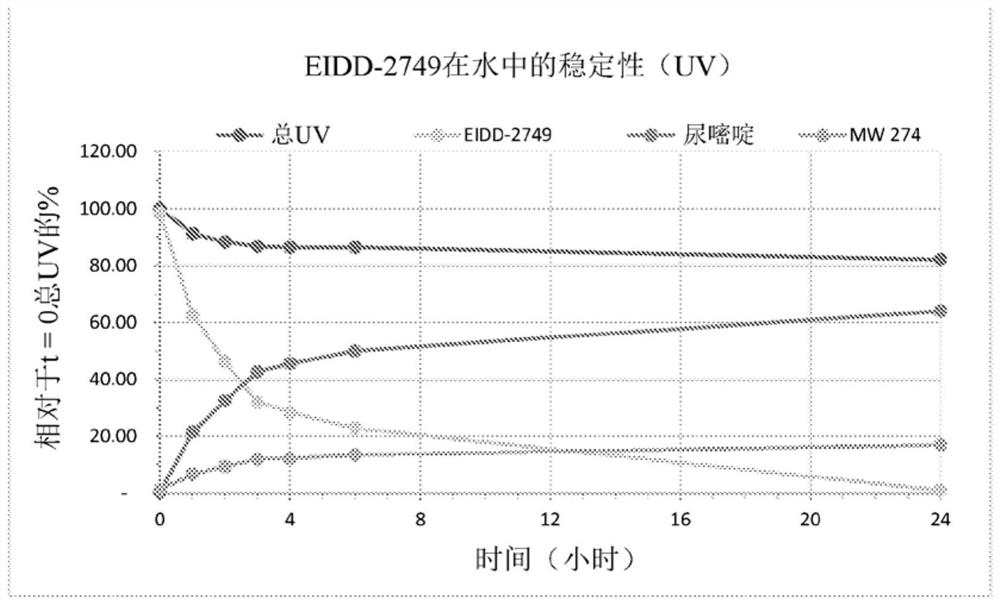
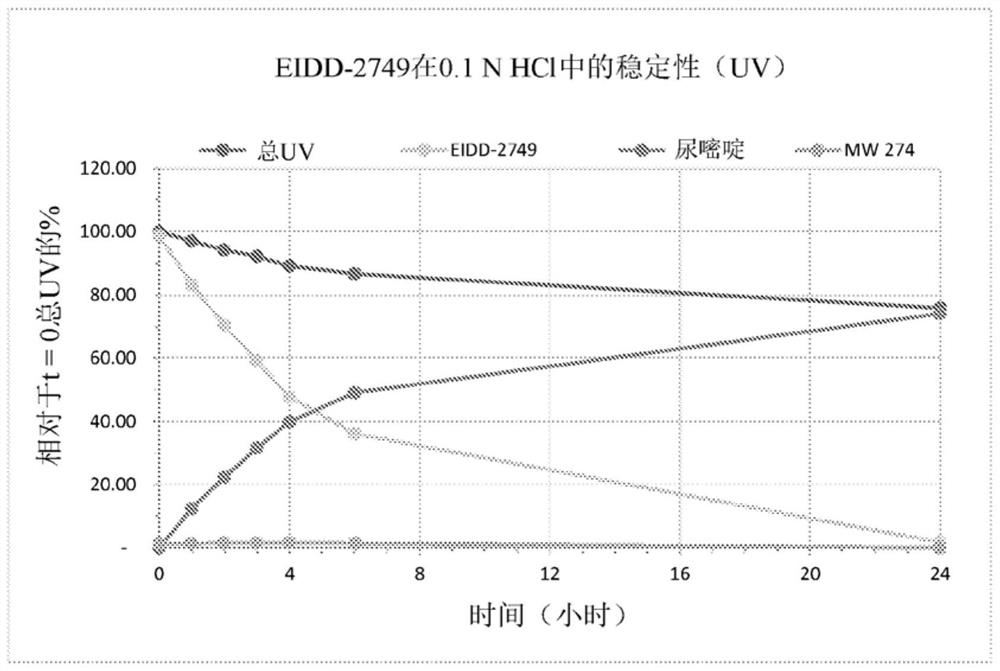
4 '-halogen-containing nucleotide and nucleoside therapeutic compositions and uses related thereto
A composition and compound technology, applied in the directions of medical preparations containing active ingredients, applications, sugar derivatives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[1894] Conjugated preparation
[1895] Monophosphate and diphosphate prodrugs have been prepared by several groups. See Jessen et al, "Bioreversible Protection of Nucleoside Diphosphates", Angewandte Chemie-International Edition English 2008, 47(45), 8719-8722, vol. The aforementioned references are hereby incorporated by reference. To prevent cleavage of the P-O-P anhydride bond, a rapidly cleaving side group (eg, bis-(4-acyloxybenzyl)-nucleoside diphosphate (BAB-NDP), which is deacylated by endogenous esterases) A negative charge is created on the diphosphate. See also Routledge et al, "Synthesis, Bioactivation and Anti-HIV Activity of 4-Acyloxybenzyl- bis(nucleosid-5'-yl) Phosphates)", "Nucleosides & Nucleotides" 1995, 14(7), 1545-1558 and Meier et al., "2',3'-dideoxy-2 Comparative study of bis(benzyl)phosphate triesters of ',3'-didehydrothymidine (d4T) and cycloSal-d4TMP--hydrolysis, mechanistic insights and anti-HIV activity (Comparative study of bis(benzyl)phosphate ...
example 2
[1899] General procedure for base coupling
[1900] The persilylation was prepared in a round bottom flask containing dry nucleobase (15.5 mmol), chlorotrimethylsilane (12.21 mmol) and bis(trimethylsilyl)amine (222 mmol) under nitrogen nucleobase. The mixture was refluxed overnight (16 hours) with stirring until all solids had dissolved. The mixture was cooled to room temperature and volatiles were removed by rotary evaporation followed by high vacuum to give the persilylated nucleobase. This compound was used immediately in the next step.
[1901] The freshly prepared persilylated nucleobase (15.50 mmol) was dissolved in 1,2-dichloroethane (50 mL) or chlorobenzene (50 mL) with stirring at room temperature under nitrogen. A solution of β-D-ribofuranose 1,2,3,5-tetraacetate (7.75 mmol) in 1,2-dichloroethane (50 mL) or chlorobenzene (50 mL) was added all in one portion to the into the stirred mixture.
[1902] To this mixture was added SnCl dropwise via syringe 4 (11.63 mm...
example 3
[1904] General Cytosine Analog Conjugation
[1905] in N 2 down, when fitted with N 4 - Bis(trimethylsilyl)amine (8.45 mmol) and ammonium sulfate (0.02 mmol) were added to a flask of benzoyl-protected cytosine analog (0.793 mmol). The flask was heated at reflux for 2 hours, and after cooling to room temperature, the solvent was removed in vacuo and further dried under high vacuum for 1 hour. The residue was dissolved in dry chlorobenzene (10 ml) and β-D- or β-L-ribofuranose 1,2,3,5-tetraacetate (0.53 mmol) was added. Then, SnCl was added dropwise 4 (0.27ml, 2.3mmol). After stirring at room temperature for 1 hour, it was heated to 60°C overnight. After cooling to 0°C, solid sodium bicarbonate (0.85 g) was added followed by EtOAc (5 mL). It was allowed to stir for 15 minutes, and then water (0.5 mL) was added slowly. The insoluble material was filtered off and washed with more EtOAc (2.5 mL). The filtrate was washed once with water, once with brine, and dried (Na 2 SO ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com