Process for production, recovery and purification of capsular polysaccharide polyribosyl-ribitol-phosphoric acid (PRP) and uses thereof
A technology of capsular polysaccharides and ribitol, applied in the field of polysaccharide purification, can solve the problems of not using tangential microfiltration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0065] While the present invention may be susceptible to different forms, preferred embodiments are shown in the accompanying drawings and the following detailed discussion, it being understood that the present embodiments are to be considered illustrative of the principles of the invention and are not intended to The invention is limited to what has been shown and described herein.
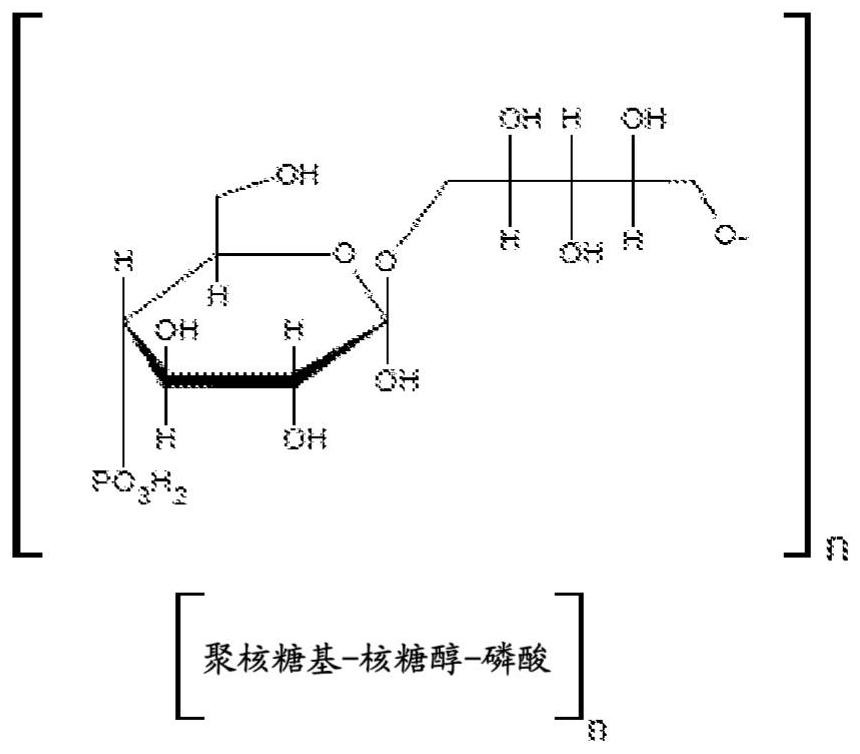
[0066] The present invention relates to a method for the production and purification of the capsular polysaccharide polyribosyl-ribitol-phosphate (PRP) produced by the bacterium Haemophilus influenzae type b (Hib). More specifically, the present invention relates to a method for the production and purification of polysaccharides (PRPs) having a purity and molecular mass suitable for subsequent chemical conjugation and production of Hib conjugate vaccines.
[0067] figure 1 The chemical structure of the polysaccharide capsule (PRP) of Haemophilus influenzae type b is shown.
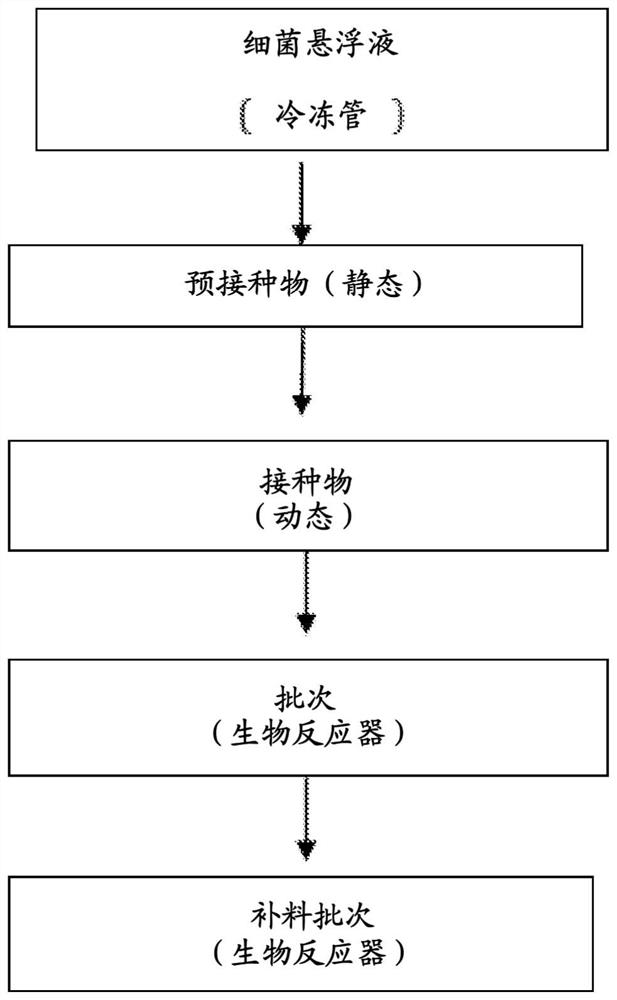
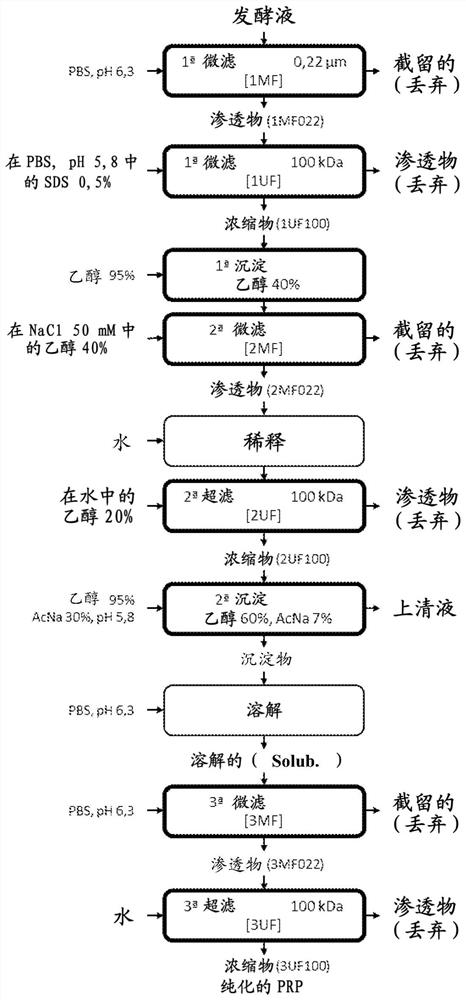
[0068] 1. Cultur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com