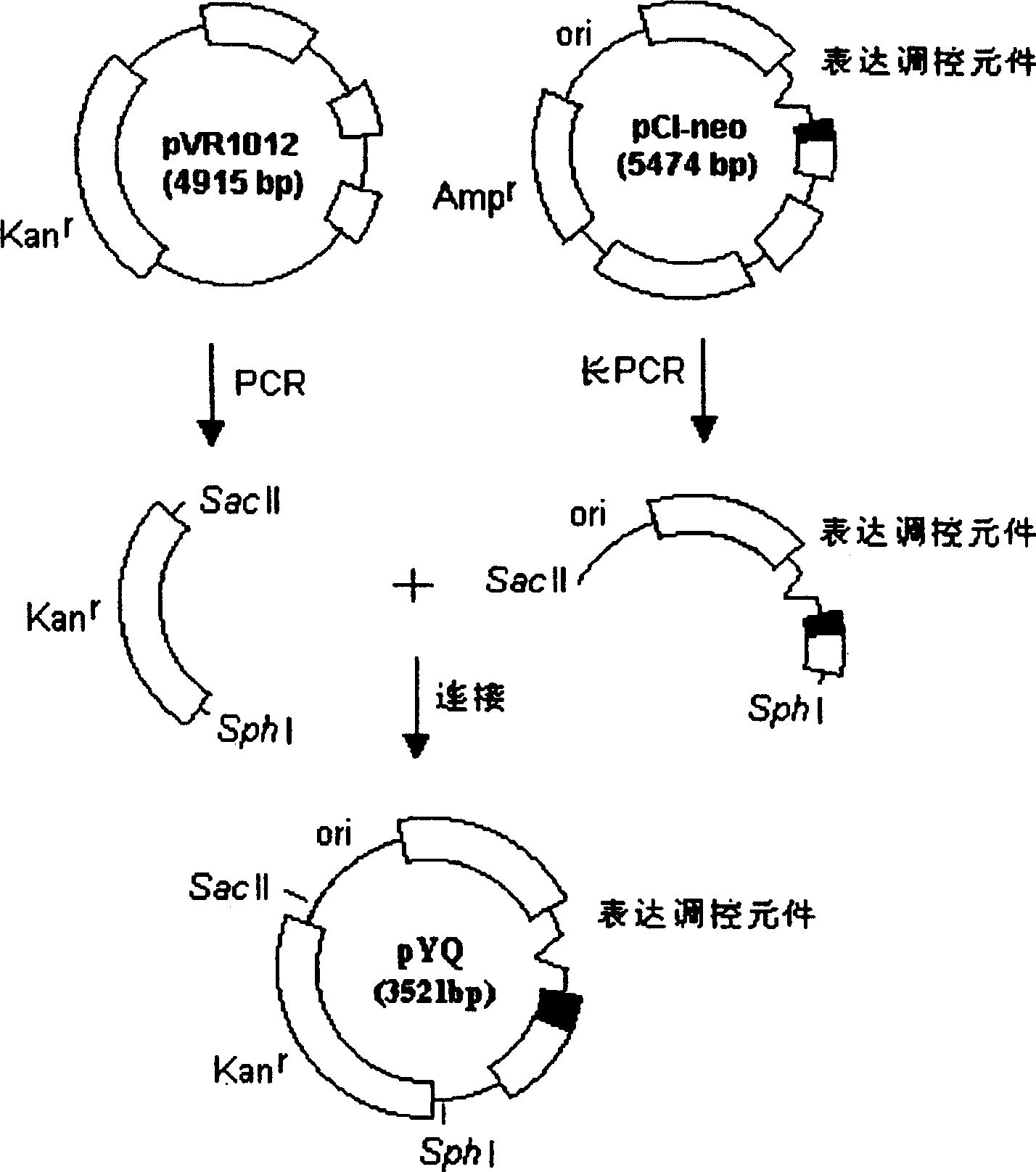
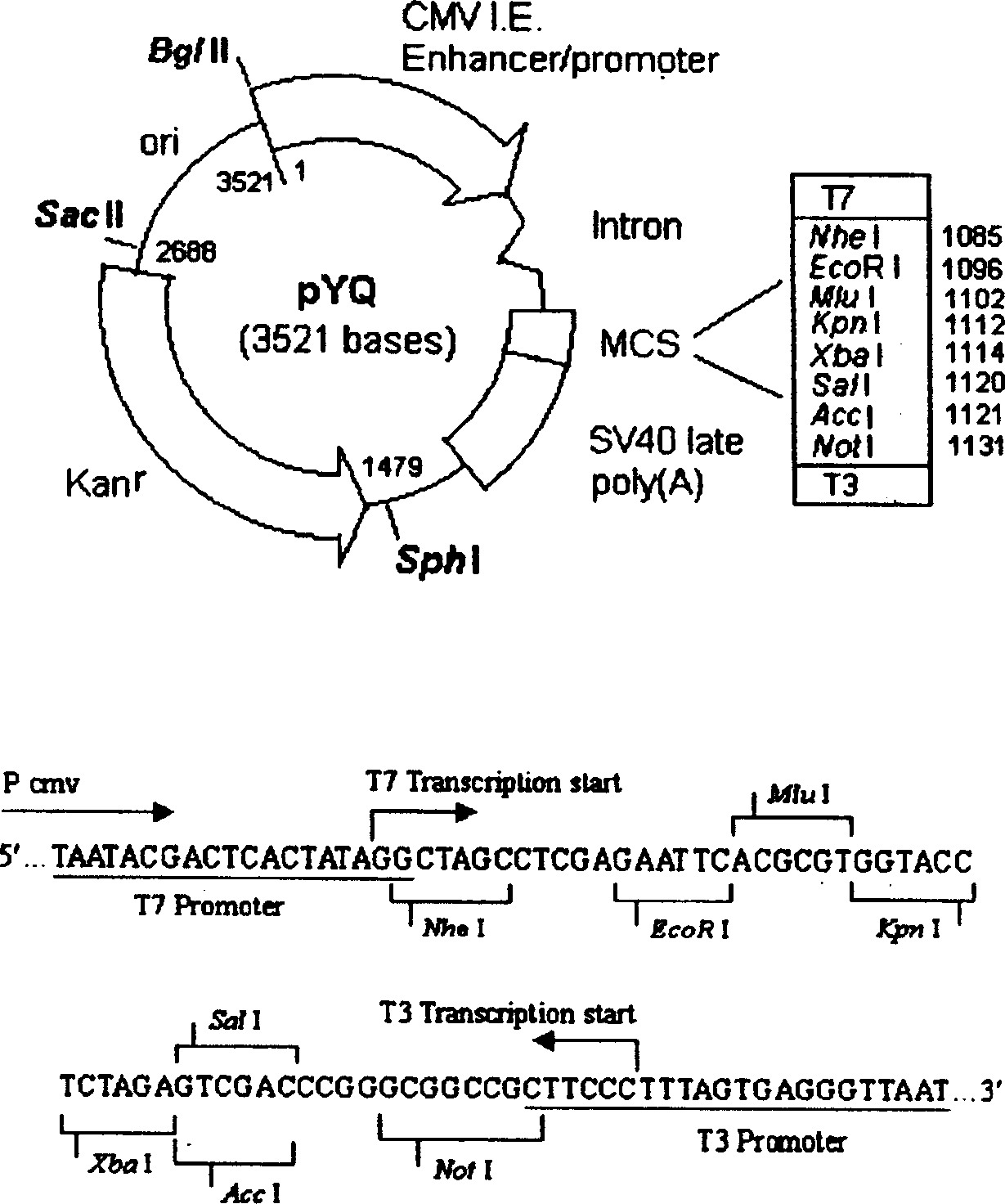
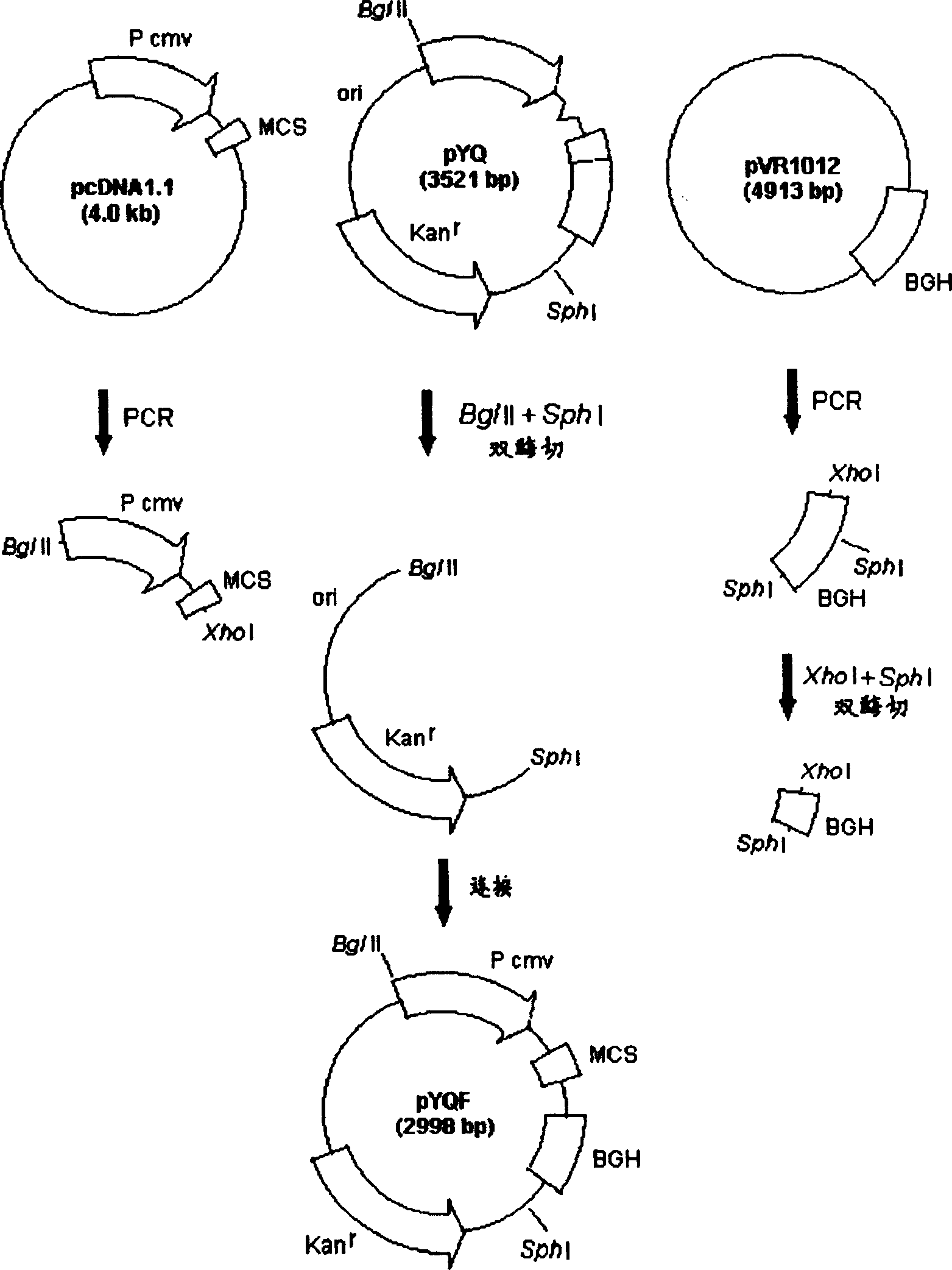
DNA vaccine carrier
A technology of DNA vaccine and carrier, applied in the field of DNA vaccine carrier construction, to achieve the effect of improving immune adjuvant effect and high copy number
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Example 1: The level of antibodies and spleen cells secreted cytokines in the body after vaccination with pYQF series vectors.
[0014] BALB / c mice were immunized with pYQF series vectors pYQF / HBs, pYQF-CpG / HBs, etc., which have been inserted into the hepatitis B virus surface antigen encoding gene. There were 12 mice in each immunization group. Using bilateral hind leg tibial anterior muscle injection method, each mouse was injected with 50μg / 100μl DNA physiological saline solution. 6 mice were immunized with pCI-neo / HBs and HBsAg protein (1μg / mouse), and an empty pYQF control and a negative mouse control (2 mice each) were set. The same dose was boosted once in the 4th week after the initial immunization. Blood was collected regularly at 2, 4, 6, and 8 weeks after the initial immunization, and the serum was separated and stored at -20°C. ELISA is also tested. The ELISA test results showed (Figures 7, 8) that pYQF / HBs, pYQF-1CpG / HBs and pYQF-2CpG / HBs can induce mice to pro...
Embodiment 2
[0017] Example 2: The level of antibodies induced by CpG ODN+HBsAg immunized mice.
[0018] HBsAg recombinantly expressed by yeast was mixed with synthetic CpG oligonucleotide (ODN) and Alum to immunize BALB / c mice, and HbsAg alone was used as a control. After 4 weeks of immunization, blood was collected, and the serum was diluted 1:50, and anti-HBs antibodies were determined by ELISA. The results show (Table 6), Oligo-1 (5′-AACGTT-3′), Oligo-4 (5′-AACGTC-3′), Oligo-5 (5′-AGCGCT-3′) and Oligo-7 ( 5'-GGCGTC-3') can increase the adjuvant effect of HBsAg inducing mice to produce specific antibodies, and has an immune adjuvant effect.
Embodiment 3
[0019] Example 3: The level of antibodies induced after pYQF-hCpG / HBs immunization.
[0020] BALB / c mice were immunized with plasmids pYQF-hCpG / HBs and pYQF / HBs, which were inserted into the gene encoding the hepatitis B virus surface antigen. 6 mice in each immunized group, each mouse was injected intramuscularly with 50 μg DNA. An empty pYQF control and a negative mouse control (2 mice each) were set up. The same dose was boosted once in the 4th week after the initial immunization. After the immune serum was diluted 1:2000 at the 8th week, the anti-HBs IgG antibody was determined by ELISA. The results show that( Figure 9) The A450 corresponding to the pYQF-hCpG / HBs immunization group was 1.12±0.47 (mean±SD, n=6), and the corresponding A450 value of the pYQF / HBs immunization group was 0.63±0.54 (mean±SD, n=6). The experimental results show that the CpG element 5'-AGCGCT-3' inserted into the pYQF / HBs vector has a certain stimulating effect on its ability to induce specific immune...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com