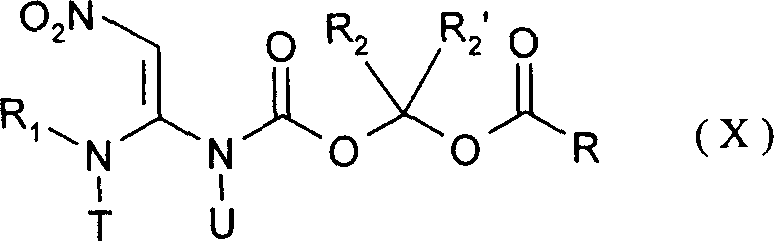
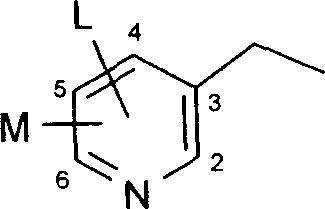
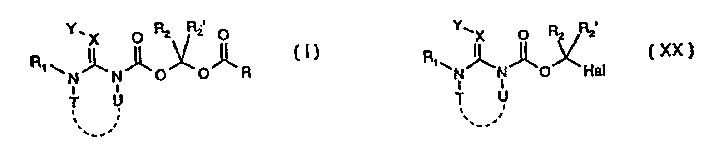Pesticides
A technology for C1-C6, compounds, applied in the field of new insecticides, which can solve the problems of treatment aversion, inability to achieve, premature stop, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0270] Preparation of primary products
[0271] Carbonic acid (chloromethyl ester) (4-nitrophenyl ester) preparation:
[0272]
[0273] A solution of 69.6 g of 4-nitrophenol and 39.6 g of pyridine in 500 ml of dichloromethane was added dropwise to a solution of 71 g of chloromethyl chloroformate in 1000 ml of dichloromethane at 0° C. over a period of 30 minutes. The reaction mixture was stirred at 0 °C for another 2 h, then 1000 ml of H 2 O. The organic phase was washed with 250 ml each of 1N NaOH and aqueous sodium chloride and dried over magnesium sulfate.
[0274] After concentrating off the solvent, 111 g of a white solid with a melting point of 61-62°C were obtained.
[0275] Preparation of Carbonic Acid (Iodomethyl Ester) (4-Nitrophenyl Ester)
[0276]
[0277] 34.8g carbonic acid (chloromethyl ester) (4-nitrophenyl ester), 45.0g NaI and 2.52g NaHCO 3 Suspended in 350 ml of acetone, the obtained suspension was stirred at a temperature of 40° C. for 16 ho...
Embodiment 1
[1095] Example 1: A tablet comprising 25 mg of a compound of general formula (I) is prepared as follows:
[1096] Element (for 1000 pieces)
[1097] The compound of general formula (I) 25.0g
[1098] Lactose 100.7g
[1099] Wheat starch 7.5g
[1100] Polyethylene glycol 6000 5.0g
[1101] Talc 5.0g
[1102] Magnesium stearate 1.8g
[1103] Demineralized water q.s.
[1104] preparation : All solid components are first sieved through a 0.6 mm mesh size sieve. Then the active ingredient, lactose, talc and half of the starch are mixed together. The other half of the starch was then suspended in 40 ml of water and the suspension was added to a boiling solution of polyethylene glycol in 100 ml of water. The obtained starch paste is added to the main batch, the mixture is granulated and water is added if necessary. The granules are dried overnight at 35°C, sieved through a 1.2 mm mesh size sieve, blended with magnesium stearate and compressed to form tablets having a m...
Embodiment 2
[1105] Example 2: Solution for Injection
[1106] Compound of general formula (I) 0.1-10%, preferably 0.5-5%
[1107] Nonionic surfactant 0.1-30%, preferably 0.5-10%
[1108] Mixture of ethanol and propylene glycol 60-99%, preferably 85-90%
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com