Ternary sulfonimine alkali salt and synthesis method thereof
A technology of sulfonimide base and synthesis method, which is applied in the field of ternary sulfonimide alkali metal salt and its synthesis, and achieves the effects of superior electrochemical stability and good electrical conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
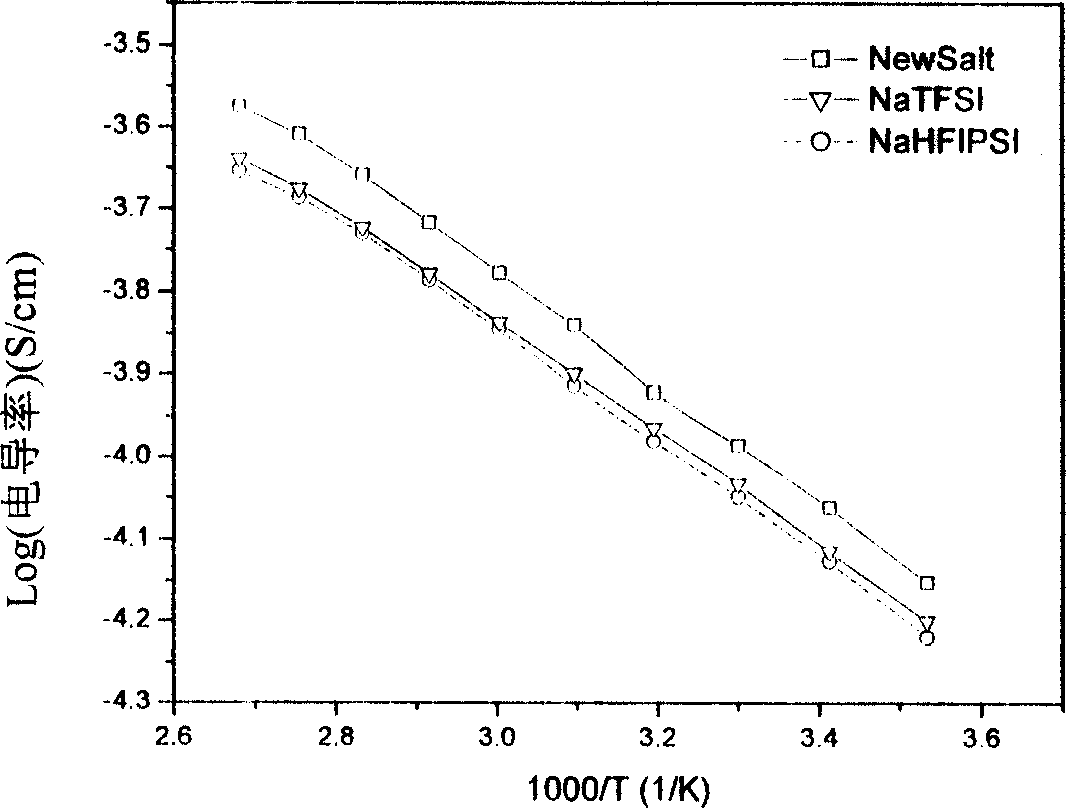
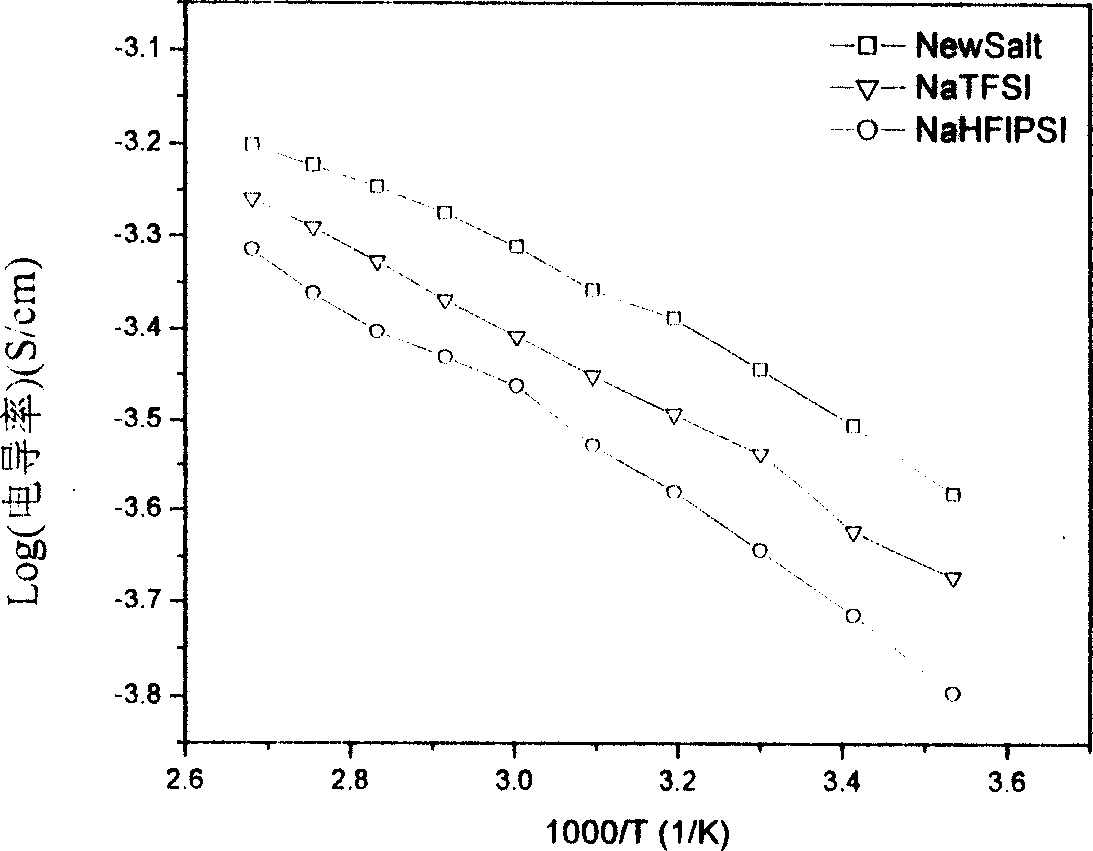
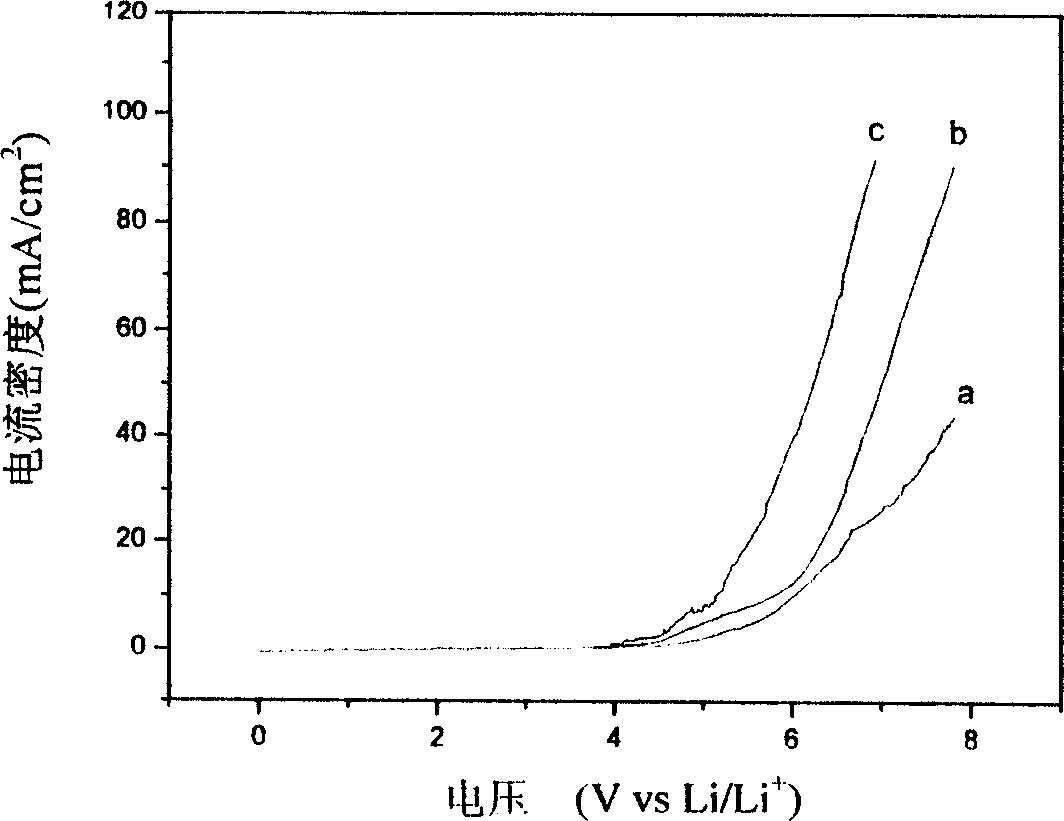
Image
Examples
Embodiment 1
[0035] Example 1: Utilize sulfonamide sodium salt to synthesize aliphatic fluorine-containing tribasic sulfonylimide sodium nitrate
[0036]
[0037] Synthetic steps:
[0038]
[0039] solvent
a
b
c
Yield
CH 3 NO 2
CH 3 CN
0.014
0.015
0.0028
0.0030
1.82
2.00
81%
83%
[0040] Structure Characterization:
[0041] FT-IR: 3000cm -1 There is no N-H vibration above, and there are obvious water peaks. The fingerprint area is also obviously different from the raw material, 995, 800, 700, 500cm in the raw material -1 The absorption peak at the point disappears in the product.
[0042] 1 H NMR: (TMS internal standard, DMSO-d 6 for the solvent) 1 The H NMR spectrum shows that there is no H atom in the compound, indicating that it does not contain raw materials and other H-containing impurities.
[0043] 19 F NMR: (CFCl 3 Internal standard, DMSO-d 6 is the solvent) has the fol...
Embodiment 2
[0048] Example 2: Utilize fluorine-containing sulfonamide to synthesize fluorine-containing ternary sulfonylimide sodium nitrate
[0049] (1) Synthesis of fluorine-containing ternary sulfonylimide nitric acid
[0050] Synthetic steps
[0051]
[0052] solvent
a
b
c
Yield
CH 3 NO 2
CH 3 CN
0.0081
0.0108
0.0162
0.0216
1.10
1.59
18%
20%
[0053] Structure Characterization:
[0054] FT-IR: 3120cm -1 There is a single N-H stretching vibration, with C 4 f 9 SO 2 NH 2 The comparison found that the characteristic peaks (946, 800, 655, 500cm) of many raw materials in the fingerprint area -1 )disappear
[0055] 1 HNMR: (TMS internal standard, DMSO-d 6 For solvent) there are three equal-area peaks at 6.46, 7.04, and 7.60ppm, which are triplets formed by H-N coupling, 1 J HN = 56Hz. No C in the spectrum 4 f 9 SO 2 NH 2 H peak (7.52ppm).
[0056] 19 F NMR: (CFCl 3 Interna...
Embodiment 3
[0063] Embodiment 3: Utilize sulfonamide sodium salt to synthesize ternary sodium sulfonimide nitrogen superacid with aromatic ring
[0064] Synthetic steps:
[0065]
[0066] solvent
a
b
c
Yield
CH 3 NO 2
CH 3 CN
0.0200
0.0168
0.0040
0.0034
2.05
1.54
70%
75%
[0067] Structure Characterization:
[0068] FT-IR: compared with sodium p-nitrobenzenesulfonamide, 990cm -1 The strong peak at disappears, and the fingerprint area is obviously different, 3300cm in the raw material -1 The peak disappears
[0069] 1 H NMR: (TMS internal standard, DMSO-d 6 There are symmetrical multiple peaks at 7.66-8.04ppm, which is the solvent, and it is a para-substituted aromatic H, and no active hydrogen peaks are found in the amine group
[0070] Elemental analysis: C (23.12%, ca.23.57%), N (11.07%, ca.11.46%), H (1.93%, ca.1.31%)
[0071] Melting point: 270°C turns black
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com