Compositions for transdermal administration of streoid drugs
A technology for steroid drug and transdermal drug delivery, which can be applied to pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 4 and comparative example 7-10
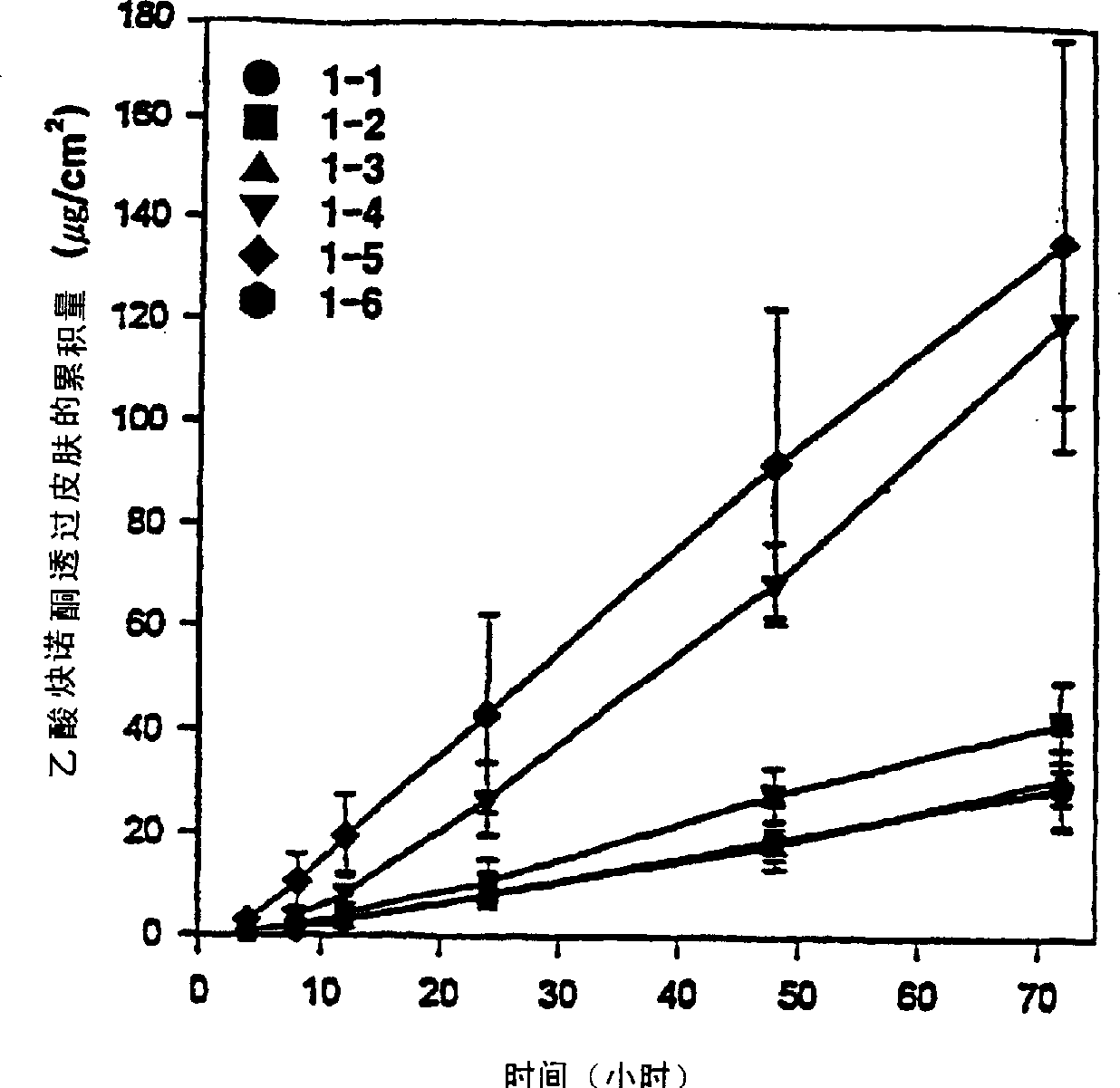
[0046] Five transdermal compositions were prepared and tested according to the method of Reference Example 1, but human cadaver skin was used, and 3.5% by weight of testosterone was used together with the absorption enhancers listed in Table 4.
[0047] serial number
[0048] As can be seen from the results in Table 4, compared with using TC or SML alone (comparative examples 8 and 9), the present invention contains diethylene glycol monoethyl ether (TC) and sorbitan monolaurate (SML) The composition in which the mixture acts as an absorption enhancer (Example 4) has a significantly higher permeation rate. Embodiment 5-7 and comparative example 11-12
Embodiment 5-7 and comparative example 11-12
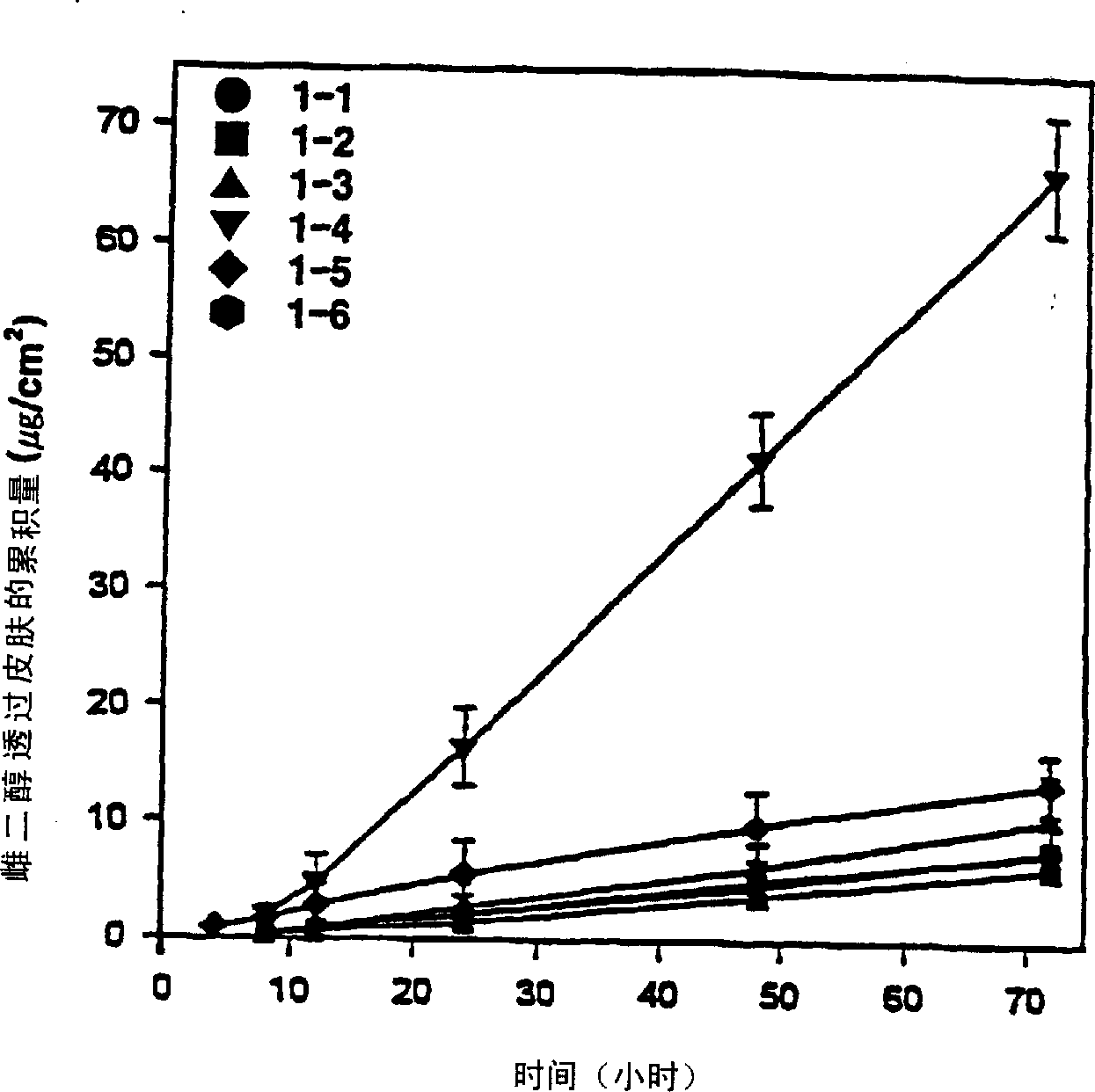
[0049] Five transdermal compositions were prepared and tested according to the method of Reference Example 1, but human cadaver skin was used, and 0.8% by weight of estradiol was used together with the absorption enhancers listed in Table 5.
[0050] serial number
[0051] As can be seen from the results in Table 5, the present invention comprises diethylene glycol monoethyl ether (TC) and sorbitan monolaurate (SML) mixture and the combination of TC and SML weight ratio in the range of 1-4 The compounds (Examples 5, 6 and 7) had significantly higher penetration rates of estradiol through human cadaver skin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com