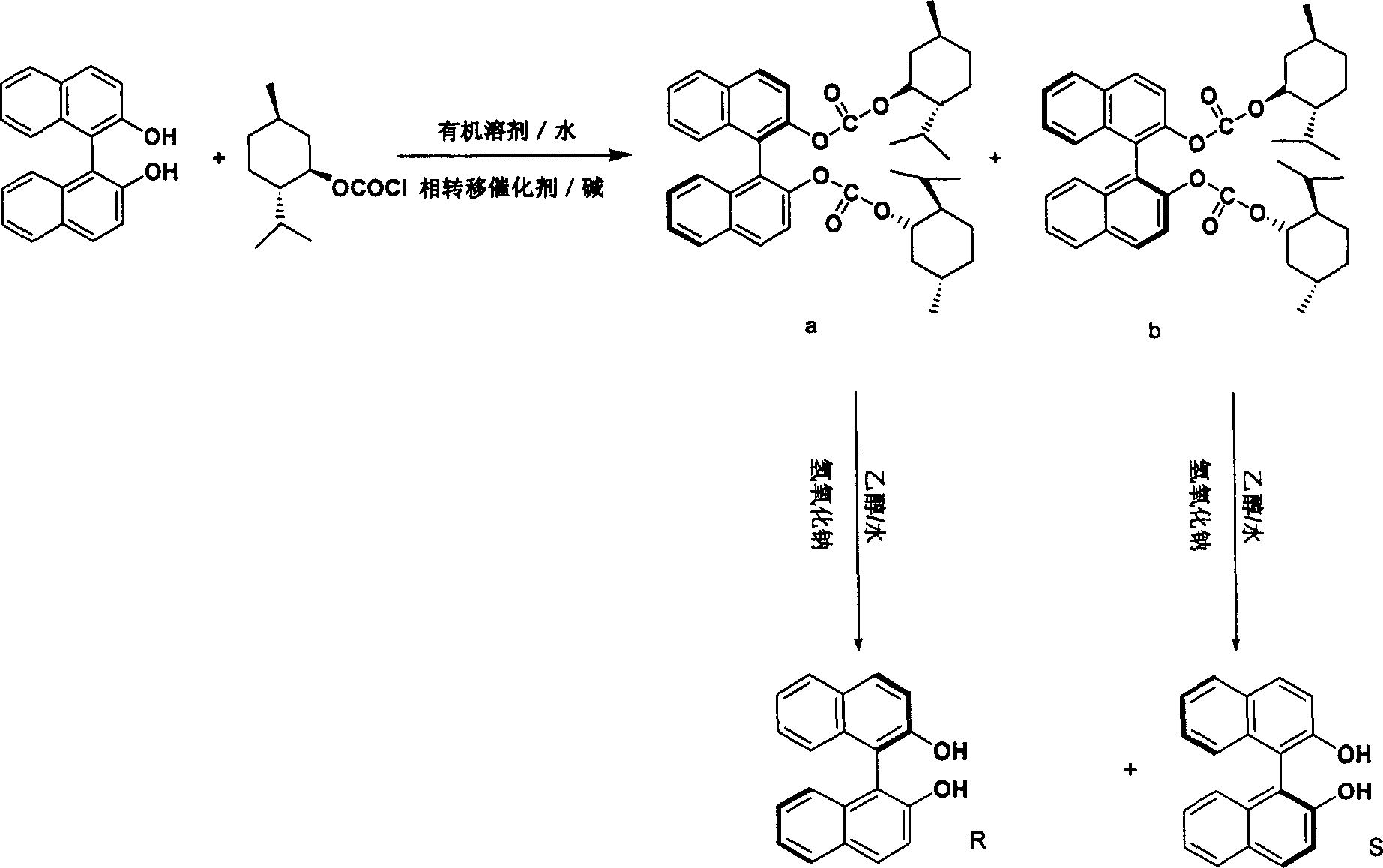
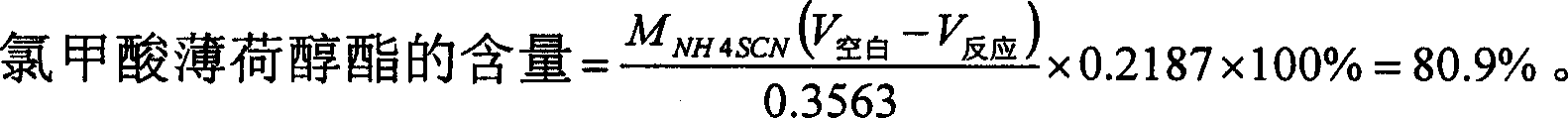Method for preparing chiral binaphthalene diol
A technology of binaphthol and chirality, which is applied in the field of preparation of chiral binaphthol, can solve the problems of high price and high purity of resolving agent, and achieve the effects of fast reaction speed, economical method and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Under stirring, dissolve 1.00 g of racemic 1,1'-binaphthyl-2,2'-diphenol in 10 ml of 6% sodium hydroxide solution, cool to room temperature, and add 0.20 g of tetrabutyl Ammonium bromide and 10.0 milliliters of dichloromethane, stir rapidly, add 1.04 milliliters of menthyl chloroformate (80.9%, 1.39 equivalents), keep room temperature and react for 8 minutes, stand still, separate the organic layer, and extract the water layer with dichloromethane , combined organic layers, washed with 3 × 10 ml of water, dried over anhydrous sodium sulfate, and removed the solvent by rotary evaporation to obtain a viscous liquid, which was dissolved with a small amount of dichloromethane and passed through a short column. Silica gel was the stationary phase, 5 cm long, 10 Wash with % ether / petroleum ether, remove the solvent, and recrystallize with 30 ml of hexane. Obtained compound 1.03 g (90.5% yield), melting point 195-198 ° C [a] 25D = -134.9, infrared [KBr, cm-1] 2958 (w), 2935 (m...
Embodiment 2
[0035] Under stirring, dissolve 2.00 g of racemic 1,1'-binaphthyl-2,2'-diphenol in 15 ml of 10% potassium hydroxide solution, cool to room temperature, and add 0.30 g of tetrabutyl Ammonium bromide and 15.0 milliliters of toluene were stirred rapidly, and 2.1 milliliters (80.9%, 1.4 equivalents) of menthyl chloroformate were added, and the reaction was kept at room temperature for 16 minutes, left to stand, and the organic layer was separated, and the aqueous layer was washed with dichloromethane 3×10 Extract in 1 ml, combine the organic layers, wash with 3×10 ml of water, dry over anhydrous sodium sulfate, filter, and remove the solvent by rotary evaporation to obtain a viscous liquid, recrystallize with 30 ml of petroleum ether at 60-90°C, concentrate the mother liquor, and pass through a short column , silica gel as the stationary phase, 5 cm long, 10% ether / petroleum ether rinse, remove the solvent, recrystallize with 30 milliliters of petroleum ether, combine the crystals,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com