Method for treatment of diarrhea disease and removing special bacterial population
A treatment method, disease technology, applied in the field of treatment of diarrheal diseases and removal of specific bacterial populations from the colon, which can solve problems such as untreatable, infection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1a
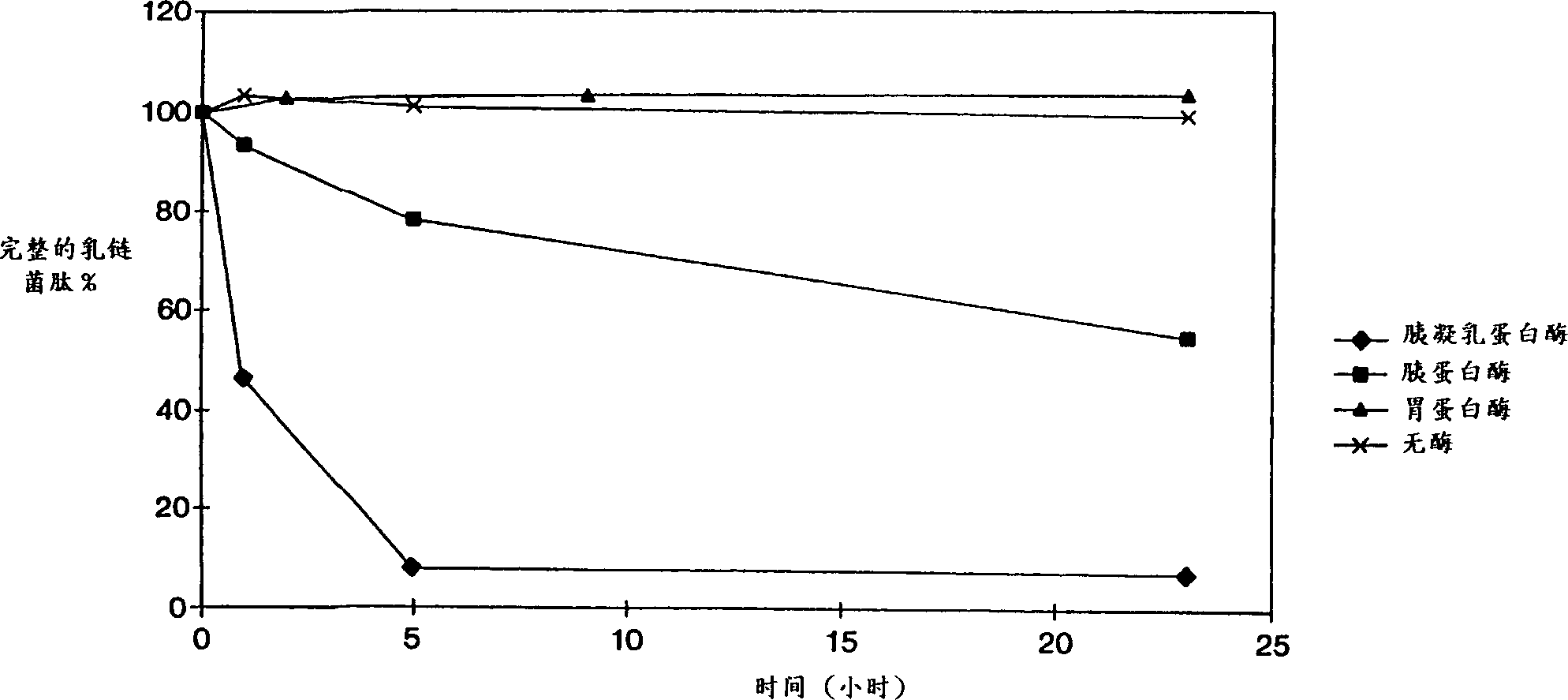
[0028] Activity of nisin against Clostridium difficile
[0029] Twenty clindamycin-resistant clinical isolates from McGuire US Veterans Administration Hospital in Richmond, Virginia were tested anaerobically on Brucella agar containing goat blood. MIC (Minimum Inhibitory Concentration) is defined as the lowest concentration tested that completely inhibits the visible growth of bacteria.
[0030] As shown in Table 1, all strains were inhibited by nisin at a concentration of 0.25 μg / ml or less.
[0031]The susceptibility of 60 clinical isolates obtained from the Hospital of the University of Florence (Italy) to nisin and to antimicrobial agents currently used in the treatment of Clostridium difficile diarrhea and colitis was tested by the same method as previously described. As shown in Table 2, the median MIC of nisin to these strains is the MIC 50 (The concentration that inhibits the growth of 50% or more of the tested strains) was 0.05 μg / ml, significantl...
Embodiment 1b
[0034] Nisin lacks activity against Bacteroides fragilis
[0035] Nine isolates of B. fragilis from McGuire US Veterans Administration Hospital in Richmond, Virginia were tested in the same manner as previously described. Even at the highest nisin concentration used (32 μg / ml), none of the strains were inhibited. Therefore, the MIC of nisin against these strains was above 64 μg / ml. Likewise, as shown in Table 3, 23 isolates from the Hospital of the University of Florence (Italy) were also less sensitive to nisin; the median MIC value was greater than 128 μg / ml.
[0036] This example shows that nisin is unlikely to disrupt the normal flora of the human intestine. It also showed that the activity of nisin alone against complex nutrient-requiring Gram-negative bacteria was not predictable from existing data on other species.
[0037]
[0038] compound
[0039] compound
[0040] All MIC values are expressed in μg / ml. Mic 50 a...
Embodiment 2
[0042] Tolerability of Nisin in Ligated Intestines of Rabbits
[0043] Five rabbits were anesthetized and kept asleep throughout the experiment. The laparotomy was performed, and a section of bowel including the ileo-cecum junction and proximal colon was ligated. Two rabbits were used as controls, and 20 mg of nisin (obtained from the manufacturer) was directly injected into the ligated intestines of the other three rabbits. Rabbits were kept for 6 hours and then slaughtered.
[0044] No signs of irritation or toxicity due to nisin treatment were found at autopsy and on histopathological examination of exposed areas of the intestine. This example shows that a single administration of nisin to the colon of rabbits at doses similar to those likely to be administered to humans does not cause any local toxicity, even when the colon is ligated to artificially maintain the dose in the colon for 6 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com