Method for the manufacture of lantibiotics
A technology of lantibiotics and cell culture, applied in peptide preparation methods, chemical instruments and methods, organic chemistry, etc., can solve problems such as complex preparation methods of lantibiotics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
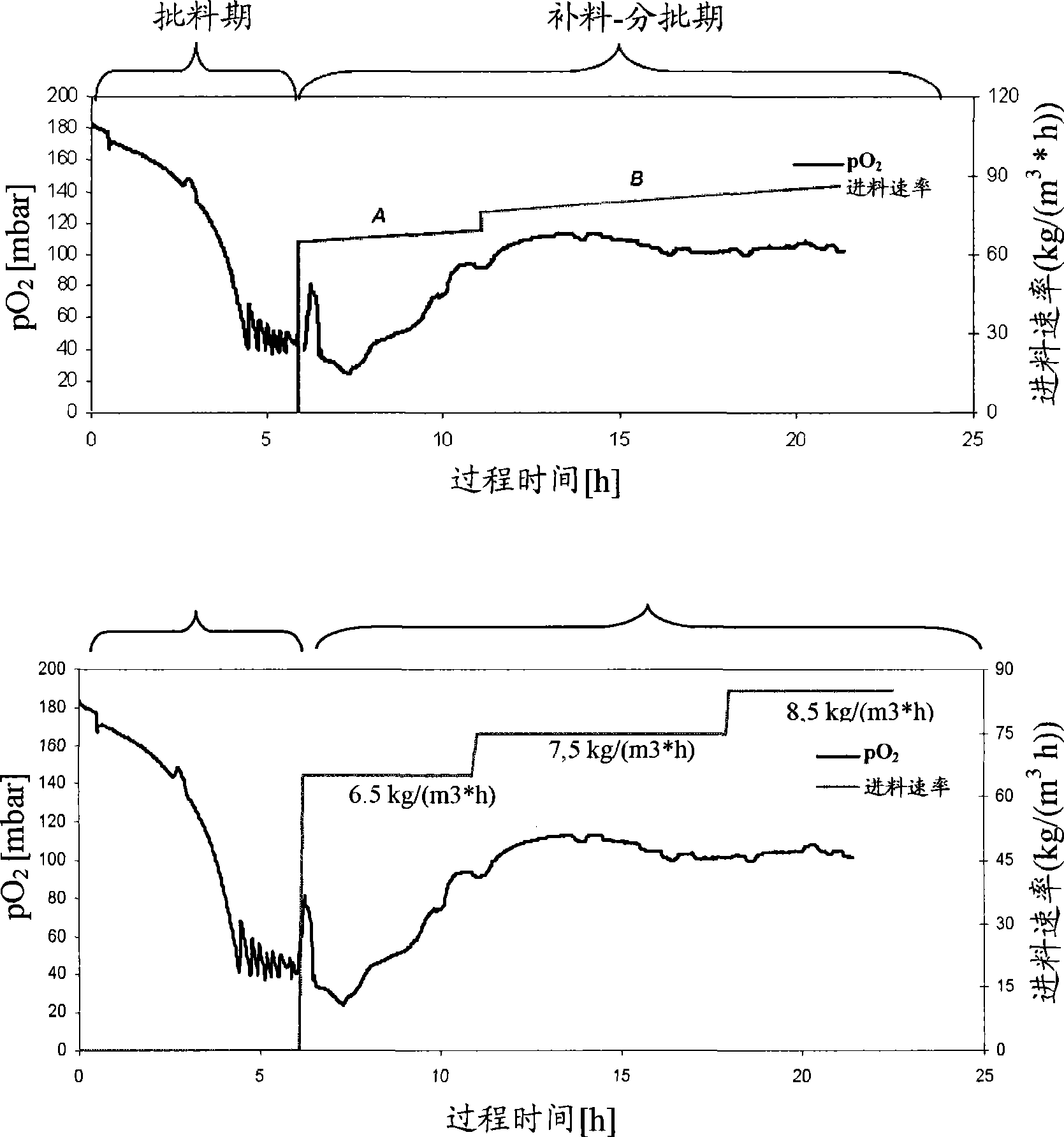
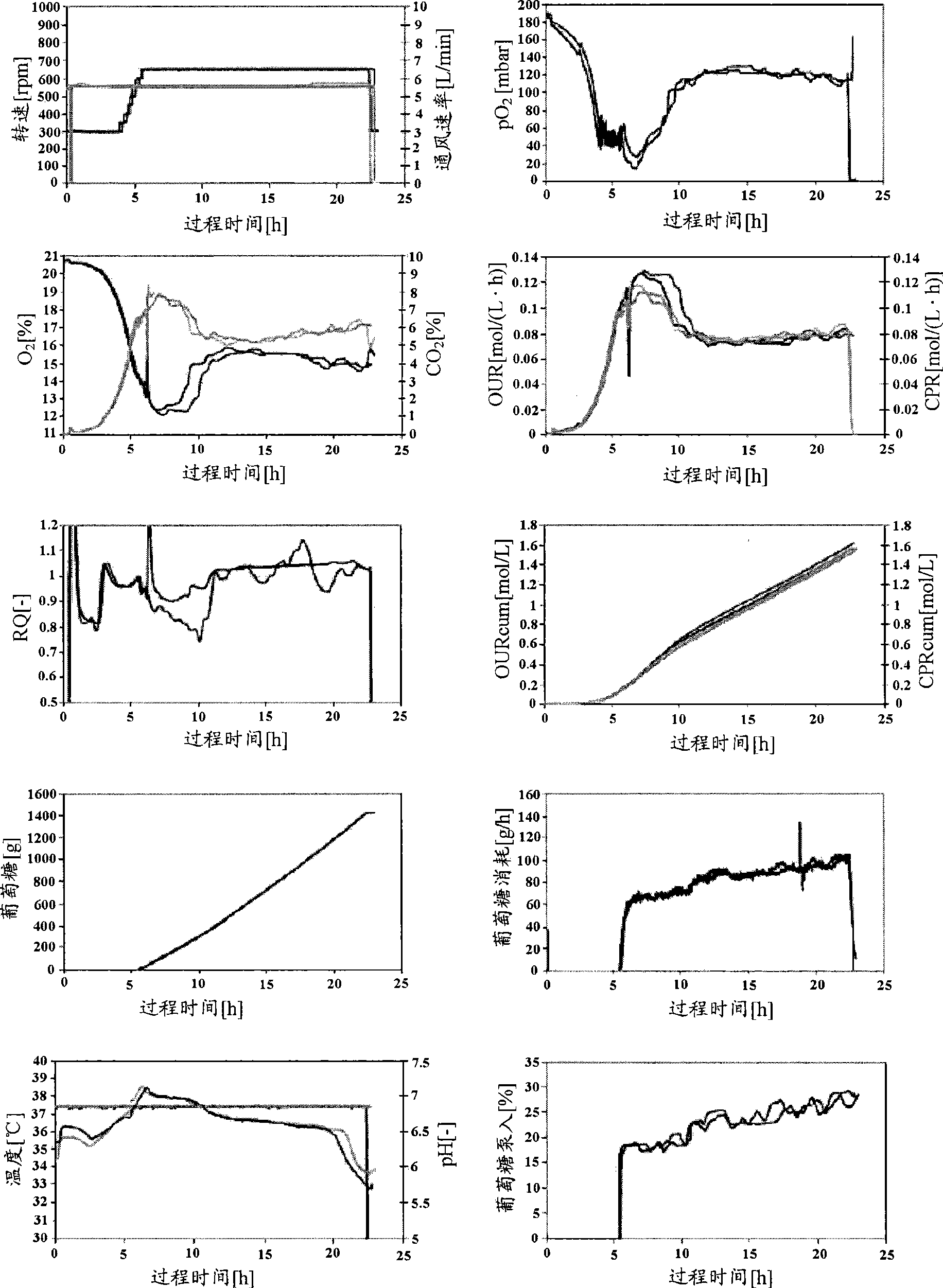
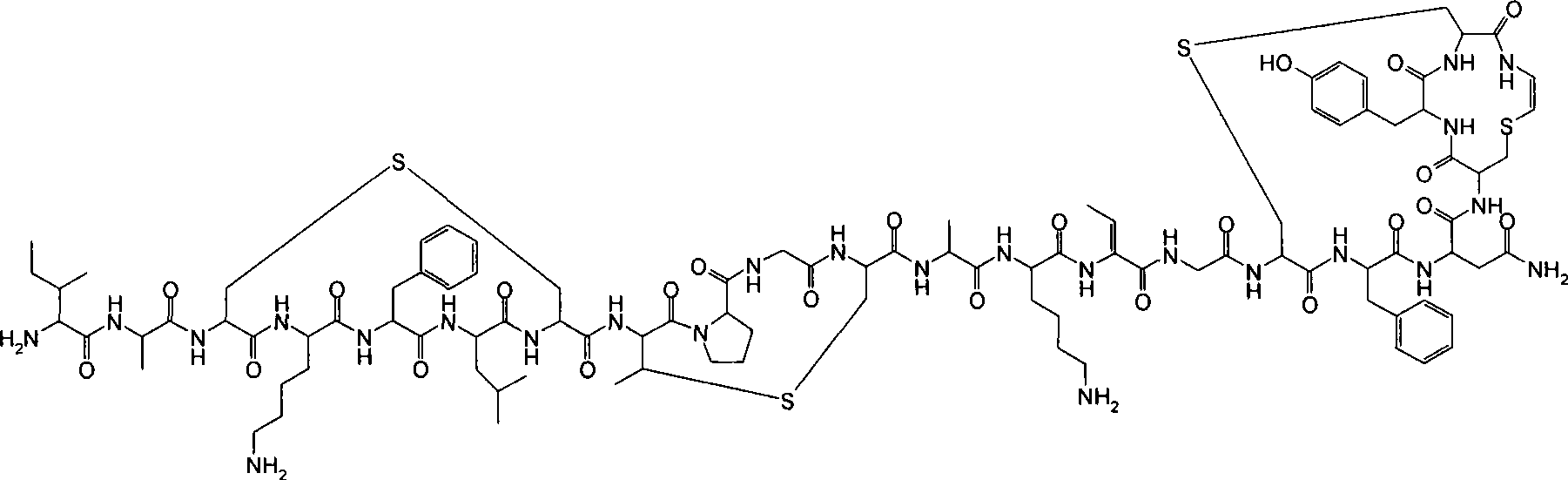
[0059] Fermentation of gallidermin
[0060] Freezing culture (WCC):
[0061] A vial of frozen stock (Zellbank BO-002; Kampagne 1940122004033, Reference 35 / 3 / 18) of Staphylococcus gallinarum (Tü 3928; DSM4616) was used to sterile inoculate the agar plates. The agar culture was incubated at 37°C for 1 day, followed by 1 day at 4°C (with Parafilm - but this step is not necessary). This culture was used to inoculate the first generation of seeds. For each production, a new vial must be used.
[0062] plate culture medium
[0063] Yeast Extract Hy-Yest 455 5g / L Kerry Bioscience Glucose x H2O 1g / L Cerestar or Merck NaCl 5g / L Fluka or Merck Bacto Agar 12g / L Bekton Dickinson h 2 o Deionization pH 7.3 (before sterilization)
[0064] Preculture or first generation seeds (2 shake flasks; 500ml):
[0065] One cultured plate (approximately 0.5 x 0.5 cm) was aseptically transferred into two 500 ml baffled shake flasks (a ba...
Embodiment 2
[0086] Purification of gallidermin
[0087] Starting from the culture supernatant of the fermentation process as described in Example 1, gallidermin was purified as follows:
[0088] Method A: Precipitation with Ammonium Sulfate
[0089] After fermentation, the cells were removed by centrifugation, and the cell-free supernatant (pH 6. 7) to precipitate the product.
[0090] In a separate experiment, precipitation with various concentrations of (saturated) ammonium sulfate was tested.
[0091] % Saturation of Ammonium Sulfate Yield(%) 20 9 30 31 40 69 50 76 60 74
[0092] The suspension was stirred slowly for another 30 minutes, then the precipitate was collected by filtration. The precipitate was dissolved in 40% ethanol, 1% aqueous acetic acid (1 / 4 of the starting volume). The yield of the product was 87% with a purity of 93.6 area % as determined by analytical HPLC.
[0093] The purity of the product can be further improved to >...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com