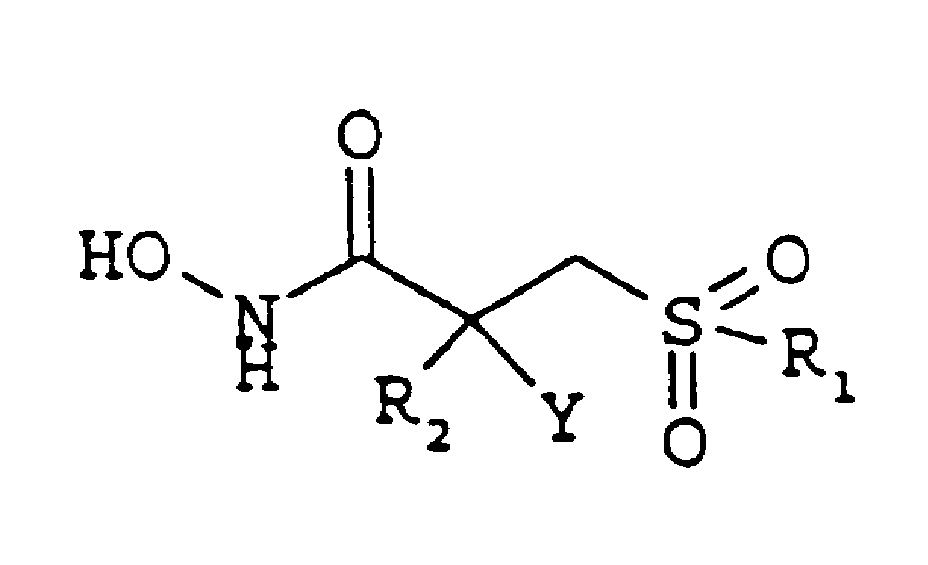
Alpha-hydroxy, amino and -fluoro derivatives of beta-sulphenyl hydroxamic acids matrix metalloproteinases inhibitors
A hydroxyl and alkyl technology, applied in the field of α-hydroxyl, amino and halogenated derivatives, can solve problems such as no tissue damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0178] It will be beneficial to further understand the compound of the present invention and its preparation in combination with the following examples, and these examples are only illustrative and do not limit the scope of the present invention. Example 1 Preparation Step 1 of N-hydroxyl-2-hydroxyl-2-[(4-methoxybenzenesulfonyl)methyl]-3-(4-phenylbenzenesulfonyl)propanamide 2-[(4- Preparation of ethyl methoxyphenylthio)methyl]acrylate
[0179] To a mixture of ethyl bromomethacrylate (1.6 g, 8.3 mmol) and 1.0 ml (8.1 mmol) of 4-methoxythiophenol in ethanol cooled in an ice bath, gradually 8 ml of 1M aqueous sodium bicarbonate solution was added dropwise. The reaction mixture was warmed to room temperature and stirred for 6 hours. The mixture was then concentrated, extracted with ethyl acetate, and washed twice with 10% aqueous hydrochloric acid and once with brine; then, it was dried over sodium sulfate and evaporated in vacuo to give a pale yellow oil. Chromatography on sil...
Embodiment 2
[0191] Chiral chromatography was accomplished with a preparative Chiralpak AD column 5.0 x 50 cm eluting with methanol at 70 ml / min. The two samples resulting from the above chromatography were separately dissolved in methanol, stirred with charcoal, filtered through celite, and evaporated to dryness to give purified enantiomer A and enantiomer B. The preparation of embodiment 2N-hydroxyl-2-hydroxyl-2-[(4-methoxybenzenesulfonyl) methyl]-3-(4-fluorobenzenesulfonyl) propanamide
[0192] Following the general procedure outlined in Example 1 (Steps 1 to 7) with non-critical changes, except that p-fluorophenylthiol was used instead of biphenylthiol in Step 3 and stirred together, the title compound was obtained. m.p.85-90°C (softening), 110-115°C (decomposition under bubbling conditions);
[0193] 1 H NMR (DMSO-d 6 )δ10.6, 8.86, 7.92-7.87, 7.75-7.72, 7.46-7.40, 7.11-7.08,
[0194] 5.64, 3.85-3.69. Preparation of Example 3 N-hydroxyl-2-hydroxyl-2-[(4-methoxybenzenesulfonyl)methy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com