Magnetic slow release 5-aminosalicylic acid with intercalate structure and its preparation method
An aminosalicylic acid and magnetic technology, which is applied in the assembly field of magnetic supramolecular intercalation structure sustained-release 5-aminosalicylic acid, can solve the problems of residual in vivo and incomplete degradation, and can shorten the treatment time and improve the treatment. effect of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1). Accurately weigh 0.012mol of Zn(NO 3 ) 2 ·6H 2 O and 0.003mol of Al(NO 3 ) 3 9H 2 O use 200ml of water to make a mixed salt solution A with a Zn / Al molar ratio equal to 4, and then add 0.0009835mol of MgFe 2 o 4 Add to mixed salt solution A;
[0039] (2). In addition, 0.038mol of NaOH and 0.008mol of 5-ASA former drug are made into mixed alkaline solution B with 100ml of water;
[0040] (3). In N 2 Slowly drop the mixed alkali B solution into the vigorously stirred mixed salt A solution under protection. After the dropwise addition, use 0.1M NaOH to adjust the final pH value to 8.4;
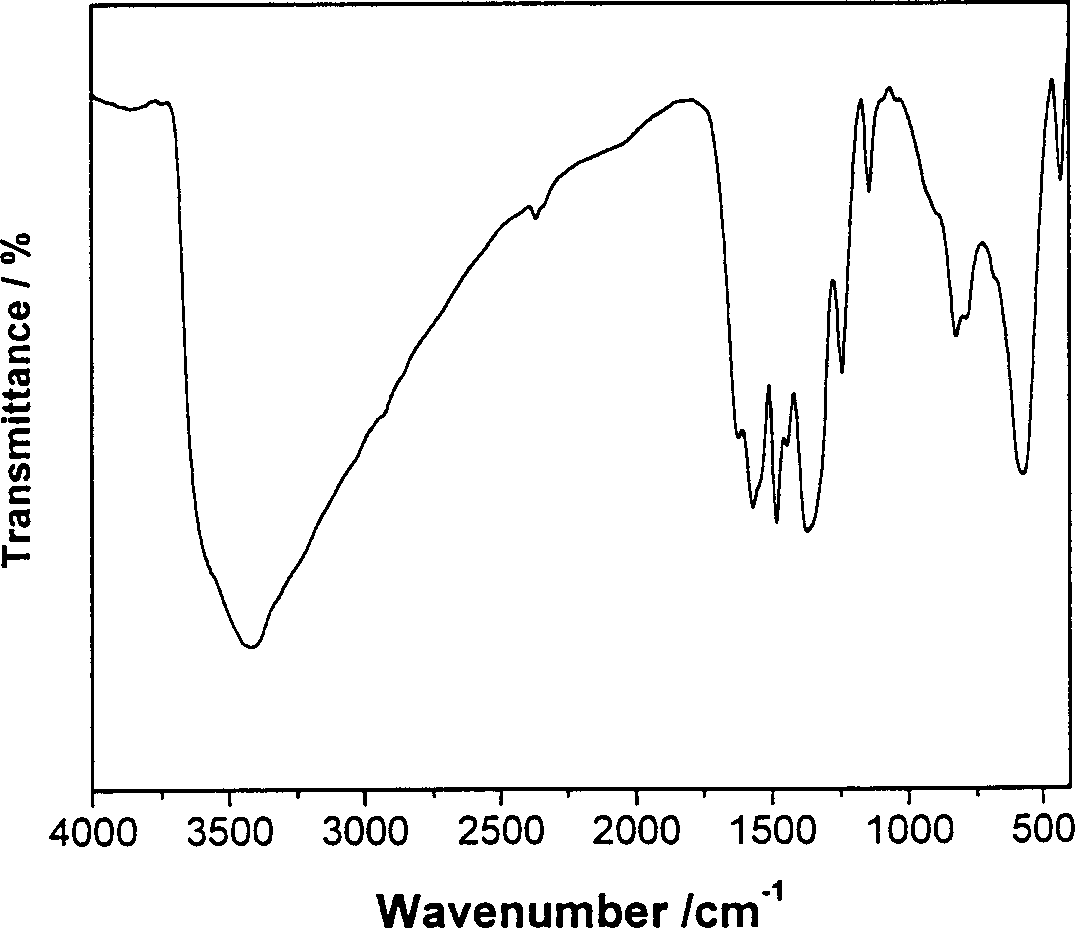
[0041] (4). The resulting slurry was crystallized at 60°C for 48 hours, filtered, washed, and dried in vacuum at room temperature for 72 hours to obtain 5-ASA-LDHs / MgFe 2 o 4 . The water used in the process is decarbonized deionized water.
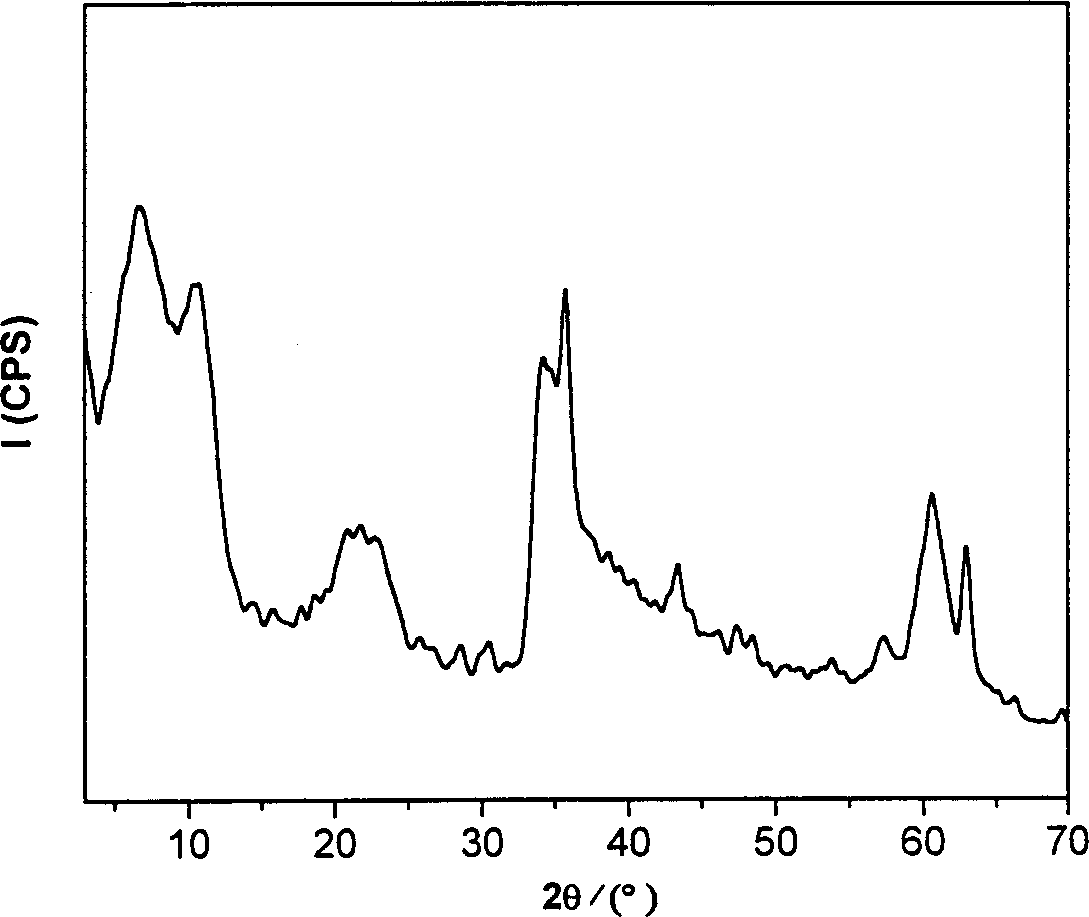
[0042] The obtained 5-ASA-LDHs / MgFe 2 o 4 Carried out X-ray powder diffraction characterization, the results are shown in figure ...
Embodiment 2
[0049] (1). Accurately weigh 0.012mol of Zn(NO 3 ) 2 ·6H 2 O and 0.003mol of Al(NO 3 ) 3 9H 2 O Use 200ml of water to make a mixed salt solution A with a Zn / Al molar ratio of 4, and add 0.00008mol of NiFe 2 o 4 ;
[0050] (2). In addition, 0.038mol of NaOH and 0.008mol of 5-ASA former drug are made into mixed alkaline solution B with 100ml of water;
[0051] (3). In N 2 Slowly drop the mixed alkali B solution into the vigorously stirred mixed salt A solution under protection. After the dropwise addition, use 0.1M NaOH to adjust the final pH value to 8.4;
[0052] (4). The resulting slurry was crystallized at 60°C for 48 hours, filtered, washed, and dried in vacuum at room temperature for 72 hours to obtain 5-ASA-LDHs / NiFe 2 o 4 . The water used in the process is decarbonized deionized water.
[0053] The resulting 5-ASA-LDHs / NiFe 2 o 4 VSM characterization was carried out, and the specific saturation magnetization of the product was 2.78emu / g.
[0054] After ana...
Embodiment 3
[0057] (1). Accurately weigh 0.008mol of Mg(NO 3 ) 2 ·6H 2 O and 0.004mol of Al(NO 3 ) 3 9H 2 O Use 200ml of water to make a mixed salt solution A with a Mg / Al molar ratio equal to 2, and add 0.0009835mol of MgFe 2 o 4 ;
[0058] (2). The NaOH of 0.038mol and the 5-ASA former drug of 0.008mol are made into mixed alkali solution B with 100ml water;
[0059] (3). In N 2 Under protection, slowly drop the mixed alkali B solution into the vigorously stirred mixed salt A solution. After the dropwise addition, use 0.1M NaOH to adjust the final pH value to 9.5;
[0060] (4). The resulting slurry was crystallized at 60°C for 48 hours, filtered, washed, and dried in vacuum at room temperature for 72 hours to obtain 5-ASA-LDHs / MgFe 2 o 4. The water used in the process is decarbonized deionized water.
[0061] The obtained 5-ASA-LDHs / MgFe 2 o 4 VSM characterization was carried out, and the specific saturation magnetization of the product was 1.76emu / g.
[0062] The empirica...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com