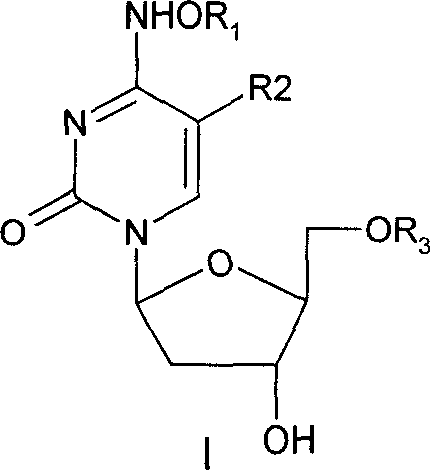
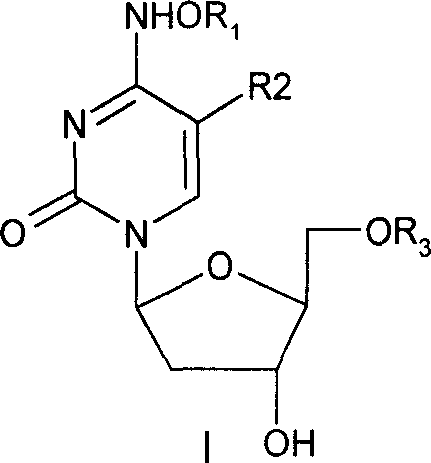
Beta-2'-deoxygenation-ramification of nucleotide, synthetic method and application of medication
A technology of derivatives and nucleosides, applied in the field of new nucleoside derivatives, can solve problems that have not been seen yet, and achieve the effects of small toxic and side effects, high anti-HBV activity, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
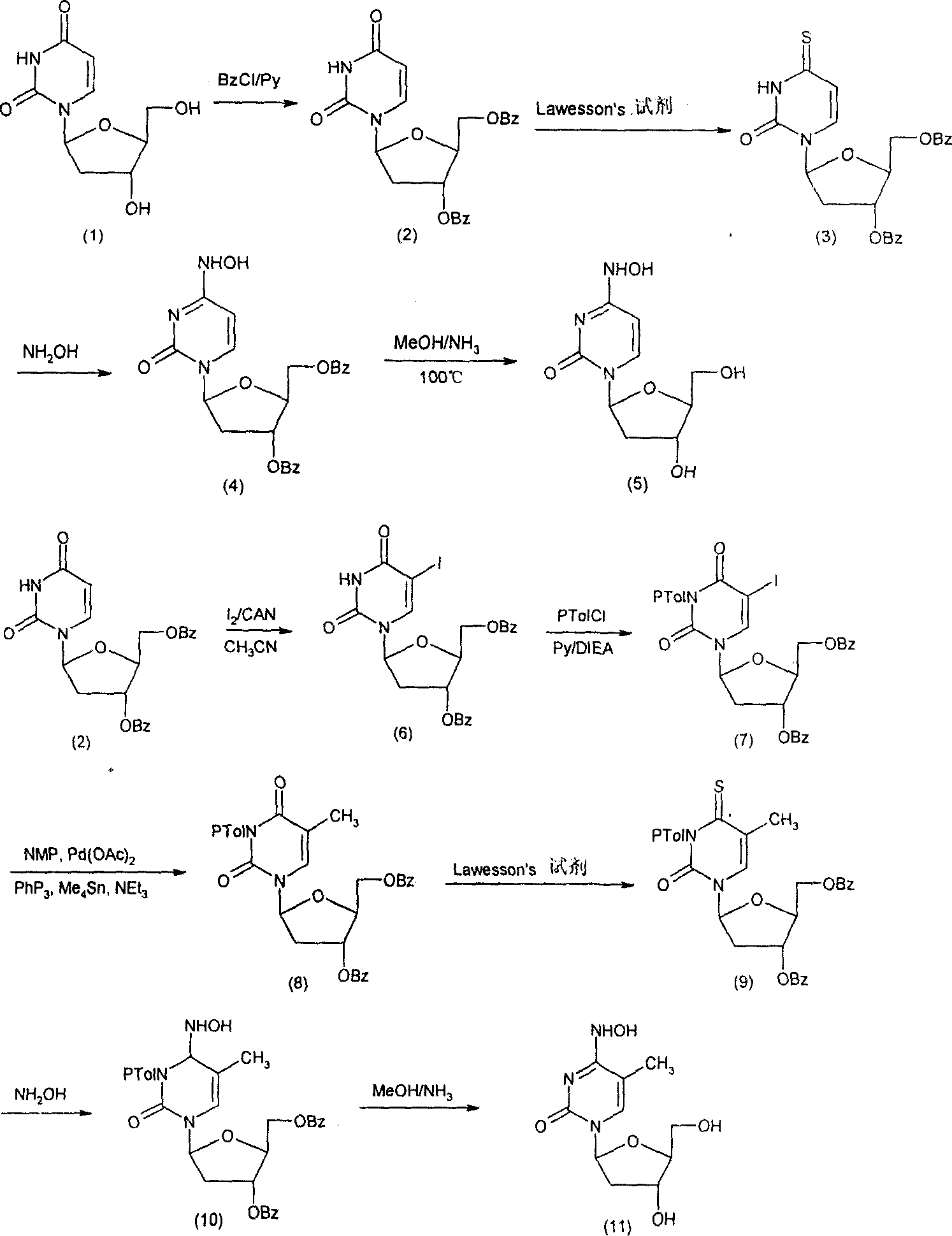
[0030] (A) Synthesis of 3',5'-dibenzoyloxy-2'-deoxy-β-L-uridine (2)
[0031] 2'-deoxy-β-L-uridine (1) (1g, 4.4mmol) was dissolved in anhydrous pyridine (20mL), and N 2 Benzoyl chloride (2 mL) was slowly added dropwise under protection. After completion, the reaction at room temperature was carried out for 6 hours. TLC detected that the starting point disappeared, and the compound 21.88 g (98.4%) was obtained by column separation, m.p.218-220°C.
[0032] 1 H NMR (CDCl 3 ): δppm 2.20-2.40 (1H, m), 2.60-2.80 (1H, m), 4.40-4.60 (3H, m), 5.62 (2H, m), 6.41 (1H, q), 7.48-8.10 (11H, m), 9.20 (1H, s, br).
[0033] (B) Synthesis of 3', 5'-dibenzoyloxy-2'-deoxy-β-L-4-thio-uridine (3)
[0034] Compound 2 (220mg, 0.5mmol) and Lawesson's reagent (408mg, 1.0mmol) were put into 1,2-dichloroethane (20mL), heated to reflux for 20h, TLC detected that the raw material point disappeared, cooled to room temperature, washed with water, concentrated and dried , column separation to obtain c...
Embodiment 2
[0042] (A) Synthesis of 3',5'-dibenzoyloxy-2'-deoxy-5-iodo-β-L-uridine (6)
[0043] Compound 4 (1g, 2.40mmol), I 2 (0.8g), cesium ammonium nitrate (CAN) (0.7g), dissolved in acetonitrile (30mL), stirred and reacted at 85°C for 5h, TLC detected that the raw material point disappeared, and cooled to room temperature to precipitate 60.9g (69.8%) of compound, m.p. 190-192°C.
[0044] 1 H NMR (CDCl 3 )δppm: 2.31 (1H, m), 2.61-2.72 (1H, m), 4.47-4.52 (1H, m), 4.68-4.71 (2H, m), 5.58 (1H, m), 6.35 (1H, q) , 7.31-8.21 (11H, m), 9.21 (1H, s).
[0045] (B) Synthesis of 3',5'-dibenzoyloxy-2'-deoxy-3-N-toluoyl-β-L-thymidine (8)
[0046] Compound 6 (1.0g, 1.78mmol) was dissolved in anhydrous pyridine (20mL), then ethyldiisopropylammonium (DIEA) (0.7mL) was added, and p-toluoyl chloride (0.8mL ), complete and then react at room temperature for 3h, TLC detects that the raw material point disappears, add a small amount of water to stop the reaction, and use CH 2 Cl 2 Extraction, w...
Embodiment 3
[0059] (A) Synthesis of 3,'5'-dibenzoyloxy-2'-deoxy-5-fluoro-β-L-uridine (13)
[0060] Carry out the same method as Example 1, except that compound 12 is used instead of compound 1 for the reaction, and the product is determined to be 3',5'-dibenzoyloxy-2'-deoxy-5-fluoro-β-L - Uridine (13), yield 96.8%, m.p. 171-173°C.
[0061] 1 H NMR (CDCl 3 )δppm: 2.31-2.42 (1H, m), 2.59-2.80 (1H, m), 4.72-4.84 (3H, m), 5.73 (1H, m), 6.28 (1H, q), 7.35-8.08 (11H, m), 9.45 (1H, s, br).
[0062] (B) Synthesis of 3',5'-dibenzoyloxy-2'-deoxy-5-fluoro-β-L-uridine (14)
[0063] Carry out the same method as Example 1, except that compound 13 is used instead of compound 2 for the reaction, and the product is determined to be 3', 5'-dibenzoyloxy-2'-deoxy-5-fluoro-β-L- 4-Thio-uridine (14), yield 98.9%, m.p.168-169°C.
[0064] 1 H NMR (CDCl 3 )δppm: 2.33-2.44 (1H, m), 2.60-2.79 (1H, m), 4.73-4.83 (3H, m), 5.81 (1H, m), 6.32 (1H, q), 7.31-8.07 (11H, m), 9.48 (1H, s, br).
[0065] (C) Synt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com