Use of Il-18 inhibitors for treatment and/or prevention of heart disease
A 1. IL-18, heart disease technology, applied in the direction of drug combination, gene therapy, cardiovascular system diseases, etc., can solve the problem that the role of IL-18 in heart disease is not described.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0091] A fully human antibody production method involves "humanization" of the mouse humoral immune system by introducing human immunoglobulin (Ig) loci into mice in which endogenous Ig genes have been inactivated, producing A mouse strain capable of producing human Ig (xenomouse). The Ig locus is complex in its physical structure and gene rearrangements, and the expression process is required to ultimately generate a broad immune response. Antibody diversity arises primarily from combinatorial rearrangements between the different V, D and J genes in the Ig locus. These loci also contain interspersed regulatory elements that control antibody expression, allelic exclusion, class switching, and affinity maturation. Introduction of non-rearranged human Ig transgenes into mice demonstrates that the mouse recombination machinery is compatible with the human gene. In addition, hybridomas secreting various isotype antigen-specific human-mouse antibodies can be obtained from heterog...
Embodiment 1
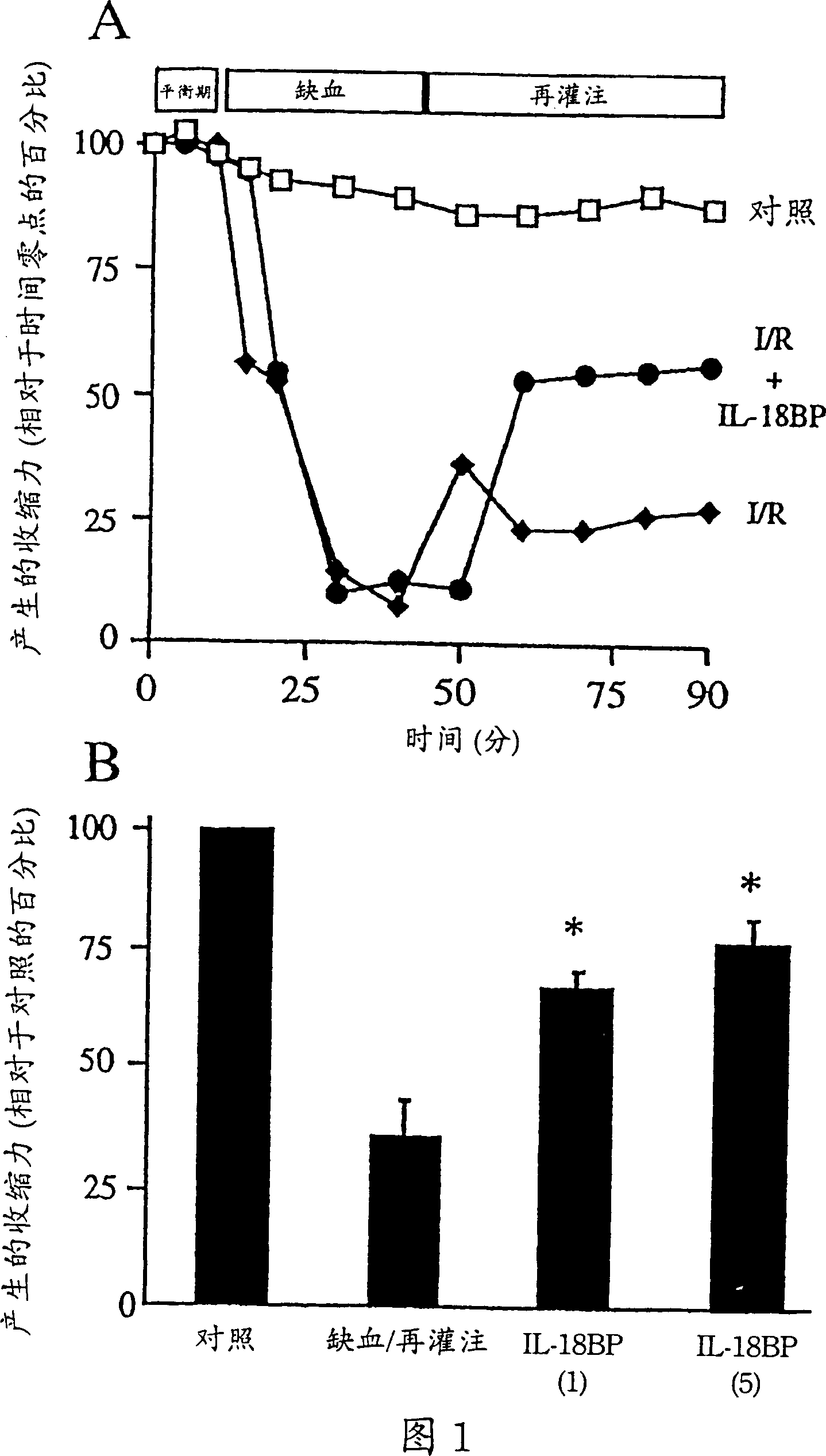
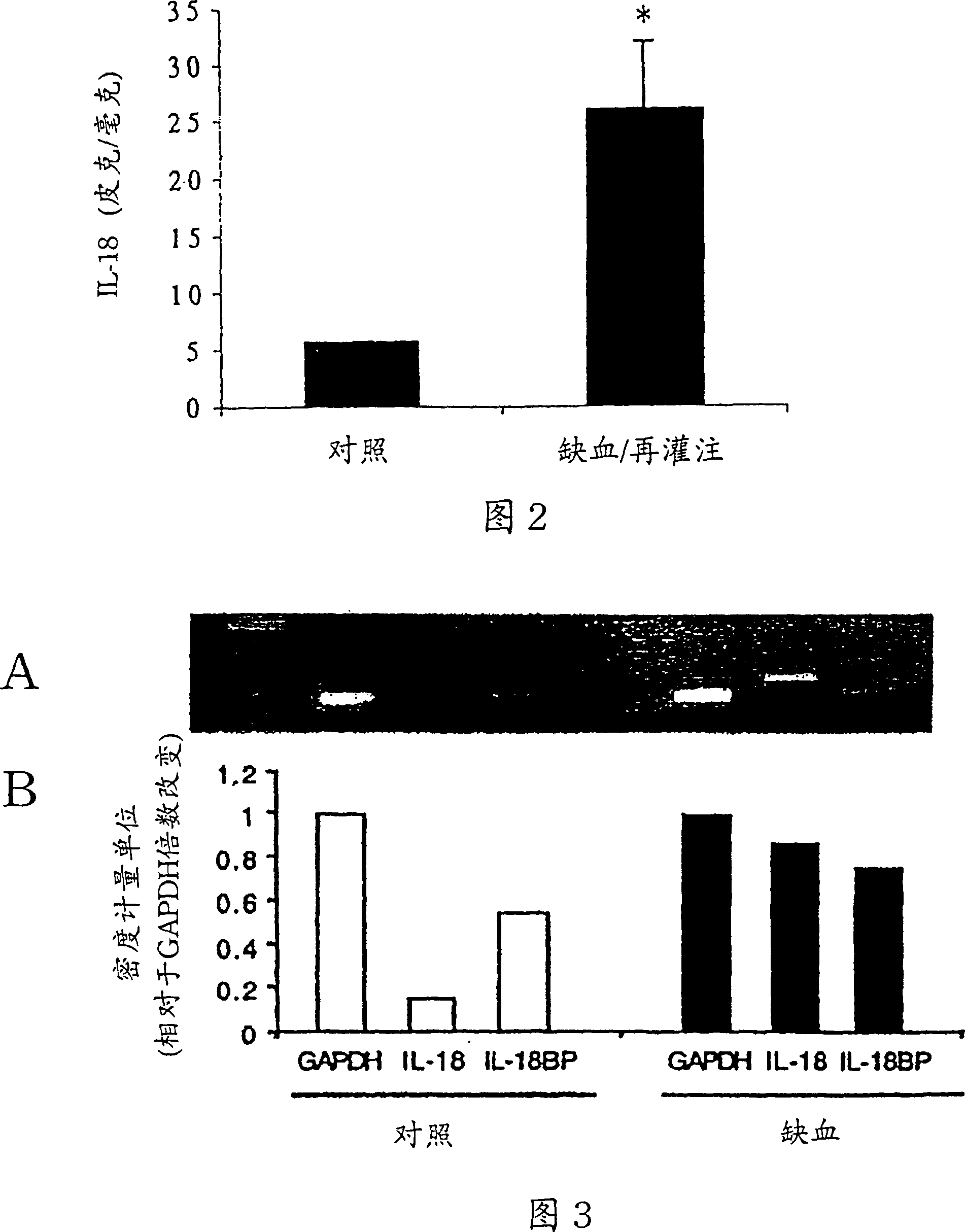
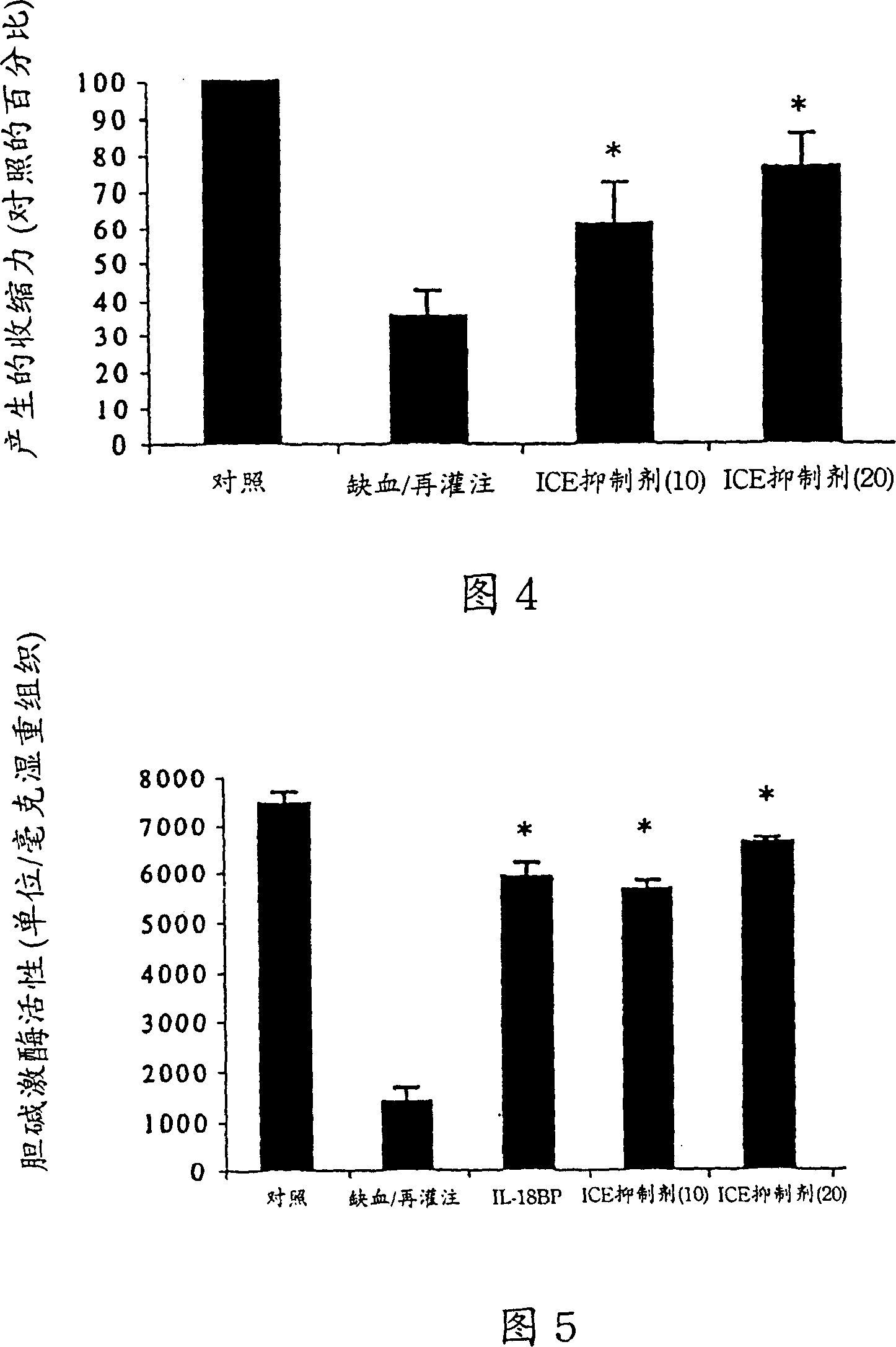
[0136] Example 1 Inhibition of IL-18 in vitro reduces myocardial ischemic dysfunction
[0137] Materials and methods
[0138] Reagent
[0139] In Chinese hamster ovary cells expressed with terminal (His) 6 The tailed IL-18BPa isoform was purified to homogeneity. It has been described that IL-18BPa-(His) 6 Ability to neutralize IL-18 (Kim et al., 2000). The ICE inhibitor (ICEi) Ac-Try-Val-Ala-Asp-chloromethanone (YVAD) was purchased from Alexis Biochemicals (San Diego) and dissolved in DMSO at a concentration of 10 mg / ml. ICE inhibitors were diluted with Tyrode's solution before use. ICEi reduced endotoxin-induced secretion of mature IL-1β from human peripheral blood mononuclear cells by 92%, as determined by ELISA (Cistron Biotechnology, Pine Brook, NJ).
[0140] isolated atrial trabeculae
[0141] Patients undergoing elective coronary artery bypass surgery with a pump oxygenator require a catheter to be inserted into the right atrium. At that time, a small segment of ...
Embodiment 3
[0173] method
[0174] In vivo intramuscular electrotransfer of mouse IL-18BP expression plasmid
[0175] C57BL / 6 mice were injected with an expression plasmid containing IL-18BP cDNA (called pcDNA3-IL18BP as described in WO01 / 85201) three times every three weeks. Control mice were injected with a control empty plasmid. The cDNA of mouse IL-18BP isoform d was isolated as described (accession number is #Q9ZOM9) (Kim et al., 2000) and subcloned into mammalian cells under the control of the cytomegalovirus promoter (Invitrogen). In the EcoR1 / Not1 site of the expression plasmid pcDNA3 in animal cells. The control plasmid is a similar construct without the therapeutic cDNA. There were 31 mice in the control group, and 27 mice in the experimental group receiving IL-18BP injection.
[0176] IL-18BP or a control expression plasmid (60 micrograms) was injected intramuscularly into the cranial tibiae of both sides of the anesthetized mice as previously described (Mallat et al., 1999...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com