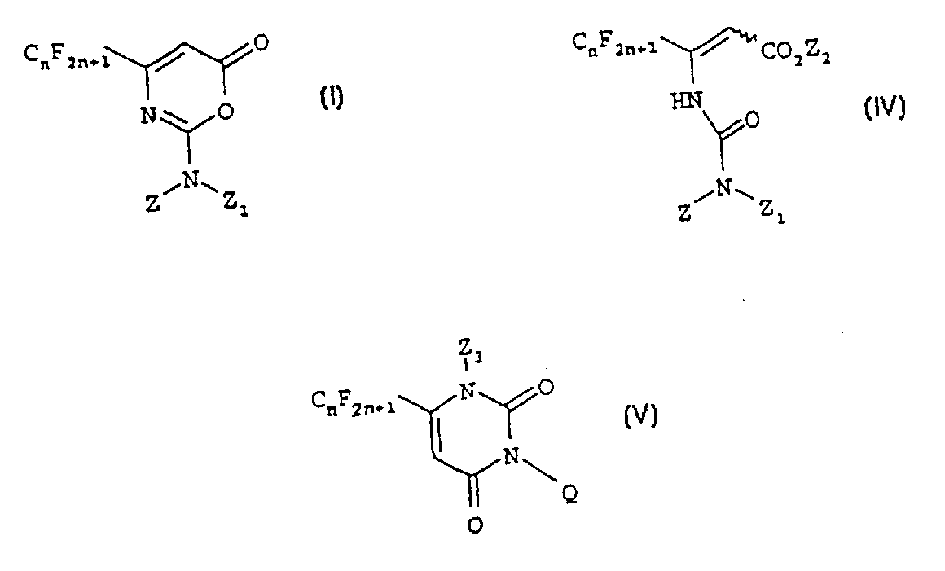
Processes and intermediates for prepn. of 1,3-diazin-b 6-ones and uracils
A kind of technology of oxazine, C1-C6
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Preparation of 3-[(N,N-dimethylcarbamoyl)amino]-4,4,4-[ethyl trifluorocrotonate, (Z)-
[0059]Add ethyl 3-amino-4,4,4-trifluorocrotonate (18.4 g, 100 mmol) to sodium hydride (60% / mineral oil, 9.6 g, 250 mmol) over 60 minutes at 5°C under nitrogen atmosphere in a stirred solution in N,N-dimethylformamide (60 mL). The reaction mixture was warmed to room temperature and held at room temperature for 15 minutes, cooled to 5°C and treated with dimethylcarbamoyl chloride (21.6 g, 200 mmol) over 60 minutes. The resulting solution was then warmed to room temperature for 2 hours, diluted with water (150 mL), and extracted with ethyl acetate (2 x 150 mL). The combined organic layers were dried, filtered and concentrated, and the mineral oil layer was removed to give a residue. The residue was purified by flash column chromatography on silica gel eluting with 85:15 hexane / ethyl acetate solution to afford the title product as a yellow liquid (18.1 g, 71% yield): 1 H NMR (DMSO-d...
Embodiment 2
[0065] Preparation of 2-dimethylamino-4-(trifluoromethyl)-6H-1,3-oxazin-6-one
[0066] 3-[N-(N,N-Dimethylcarbamoyl)amino]-4,4,4-trifluorocroton was treated with phosphorus pentachloride (4.16 g, 0.02 mol) in 3 batches at 15 min intervals A solution of ethyl acetate (5.08 g, 0.02 mol) in phosphorus oxychloride (3 mL) was stirred for 30 minutes and treated with ice and water. The resulting aqueous mixture was extracted with ethyl acetate. The organic layer was washed sequentially with saturated sodium bicarbonate and water and evaporated to give the title product as a white solid (3.9 g, 93.8% yield), 1 H and 19 F NMR confirmed that it was identical to a sample prepared by a literature method (Bull. Soc. Chem. Belg. 101, 313, 1992). The title product was further purified by recrystallization from heptane (74.5% crystallization yield).
[0067] Using essentially the same method, but replacing 3-[N-(N,N- Dimethylcarbamoyl)amino]-4,4,4-trifluorocrotonate, 2-diethylamino-4-(t...
Embodiment 3
[0069] Preparation of 3-isopropyl-6-(trifluoromethyl)-2,4(1H,3H)-pyrimidinedione
[0070] Isopropylamine (1.2 g, 20.3 mmol) and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU, 6 mL) were added to 2-dimethylamino-4-tris Fluoromethyl-6H-1,3-oxazin-6-one (4.0 g, 19.2 mmol) in solution in xylene (34 mL). The resulting reaction mixture was heated at 100 °C for 3 hours, cooled, washed with 5 wt.% HCl (40 mL), and extracted with ethyl acetate. The combined organic phases were concentrated and triturated with heptane. The solid was filtered off and dried to afford the title product as a yellow powder (2.3 g, 54% yield, mp 127-129°C), which 1 H NMR (DMSO-d 6 ) δ 6.11(1H, s), 4.96(1H, sp), 1.35(6H, d); and 19 F NMR δ-68.9(s) confirmed it to be the desired product.
[0071] Using essentially the same procedure, but using the appropriate amine, the following compounds were obtained:
[0072] Q acid / base solvent mp(°C) yield (%)(S)-(+)-CH(CH 3 )C 2 h 5 DBU Toluene 92-95 27(R)-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com