Method for separating cells using immunorosettes
A rose flower and cell technology, applied in immunoglobulin, chemical instruments and methods, biochemical equipment and methods, etc., can solve problems such as time cell loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 4
[0063] The preparation of embodiment 1 tetramer
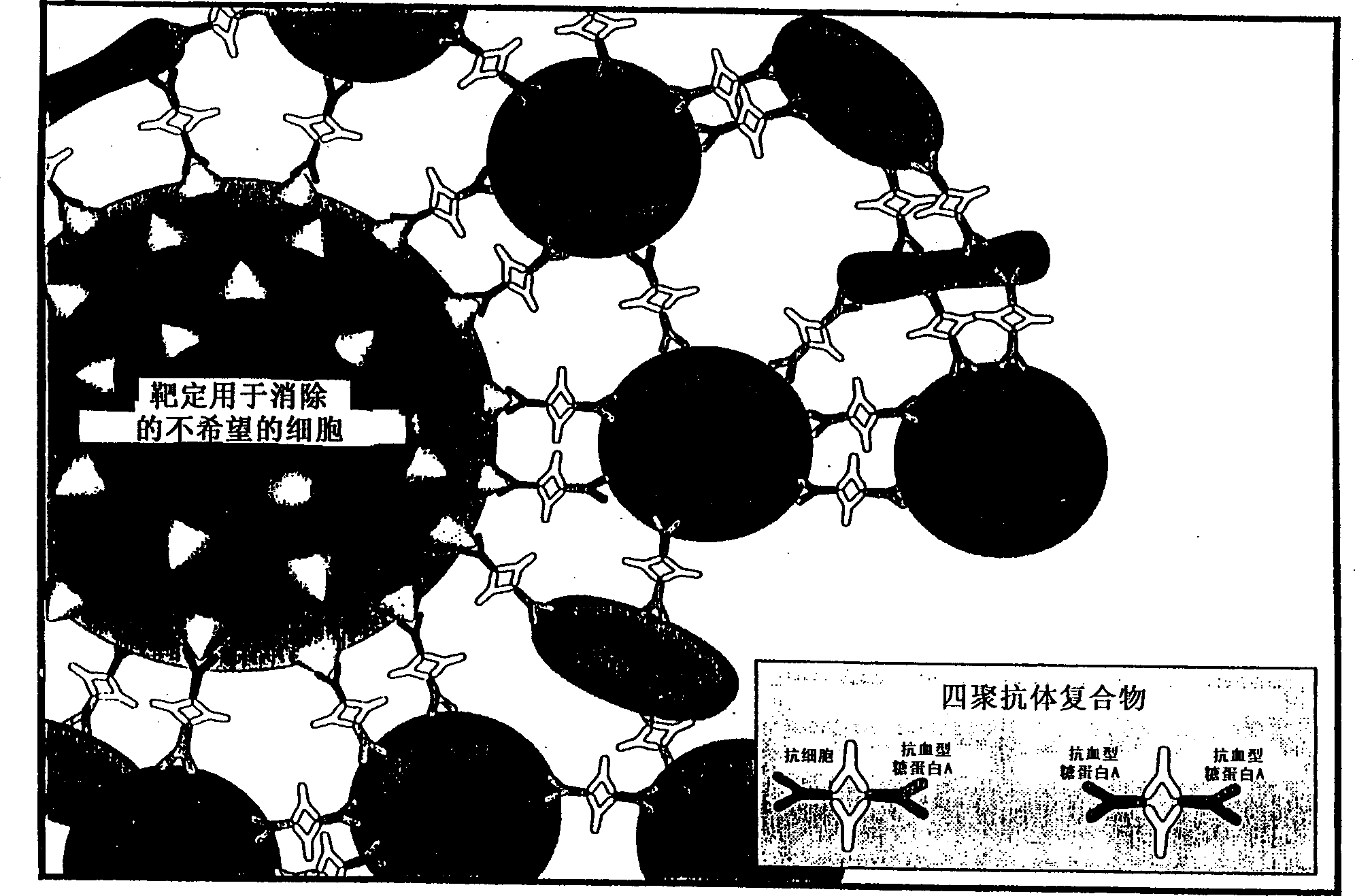
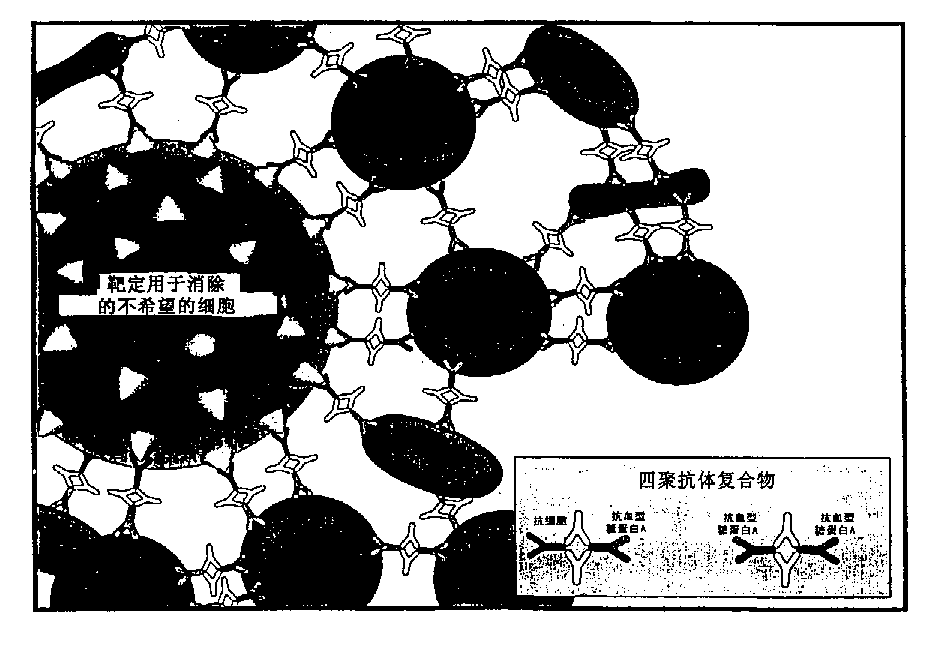
[0064] To prepare tetrameric antibody complexes for use in the methods of the invention, the following protocol can be used: (a) 1 mg of cell-specific antibody rosetting (e.g., anti-CD2, CD3, CD4, CD8, CD14, CD16, CD19, etc.); (b) add 3 mg anti-glycophorin A antibody (anti-erythrocyte), mix well; (c) then add 4.0 mg P9 antibody or 2.72 mg P9 F(ab') 2 Antibody fragments. Incubate overnight at 37°C. The P9 antibody binds to the Fc portion of the antibody added in steps (a) and (b) to form a tetrameric antibody complex. For more information on tetramer preparation, see US Patent No. 4,868,109 to Lansdorp, incorporated herein by reference. Tetrameric antibody complexes containing different antibodies against expressed antigen or nucleated cells are prepared separately and then mixed.
[0065] Antibody compositions are prepared by combining different tetrameric antibody complexes depending on which cells one wishes to exclude. ...
Embodiment 2
[0066] Example 2 Immune rosetting method using Ficoll
[0067] The following describes a negative selection method for immune rosetting of cells from whole peripheral blood using Ficoll Hypaque.
[0068] 1. Add 100 μL of antibody composition per mL of whole peripheral blood.
[0069] 2. Incubate at room temperature for 20 minutes.
[0070] 3. Dilute the sample with an equal volume of phosphate buffered saline (PBS) + 2% fetal calf serum (FCS) and mix gently.
[0071] 4. Layer the diluted sample over the Ficoll Hypaque, or layer the Ficoll under the diluted sample.
[0072] 5. Centrifuge at 1200 x g for 20 minutes at room temperature with the brake turned off.
[0073] 6. Remove enriched cells from the Ficoll:plasma interface.
[0074] 7. Wash the enriched cells with 5-10 x volume of PBS + 2% FBS.
[0075] Note: To enrich for monocytes and other adherent cells, add 1 mM EDTA to whole blood samples and all wash / dilution solutions.
Embodiment 3
[0076] Example 3 Method for Immuno-Rosette Formation Utilizing Hetastarch Precipitation
[0077] The following describes a negative selection method for immune rosetting of cells from whole peripheral blood using hydroxyethyl starch. Hetastarch is one of many compounds that increase the rate of red blood cell sedimentation through agglutination.
[0078] 1. Add 1 mL of 6% hydroxyethyl starch saline solution per 5 mL of blood and mix.
[0079] 2. Add the antibody composition described in Example 1 to whole blood so that the final concentration of each anti-nucleated cell antibody is 1.0-2.0 μg / mL.
[0080] 3. Incubate at room temperature for 10 minutes.
[0081] 4. Centrifuge at 50 x g for 5 minutes at room temperature.
[0082] 5. Remove the supernatant. This fraction contains enriched cells.
[0083] 6. Wash the enriched cell fraction with 2-5x volumes of PBS + 2% fetal bovine serum (FBS).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com