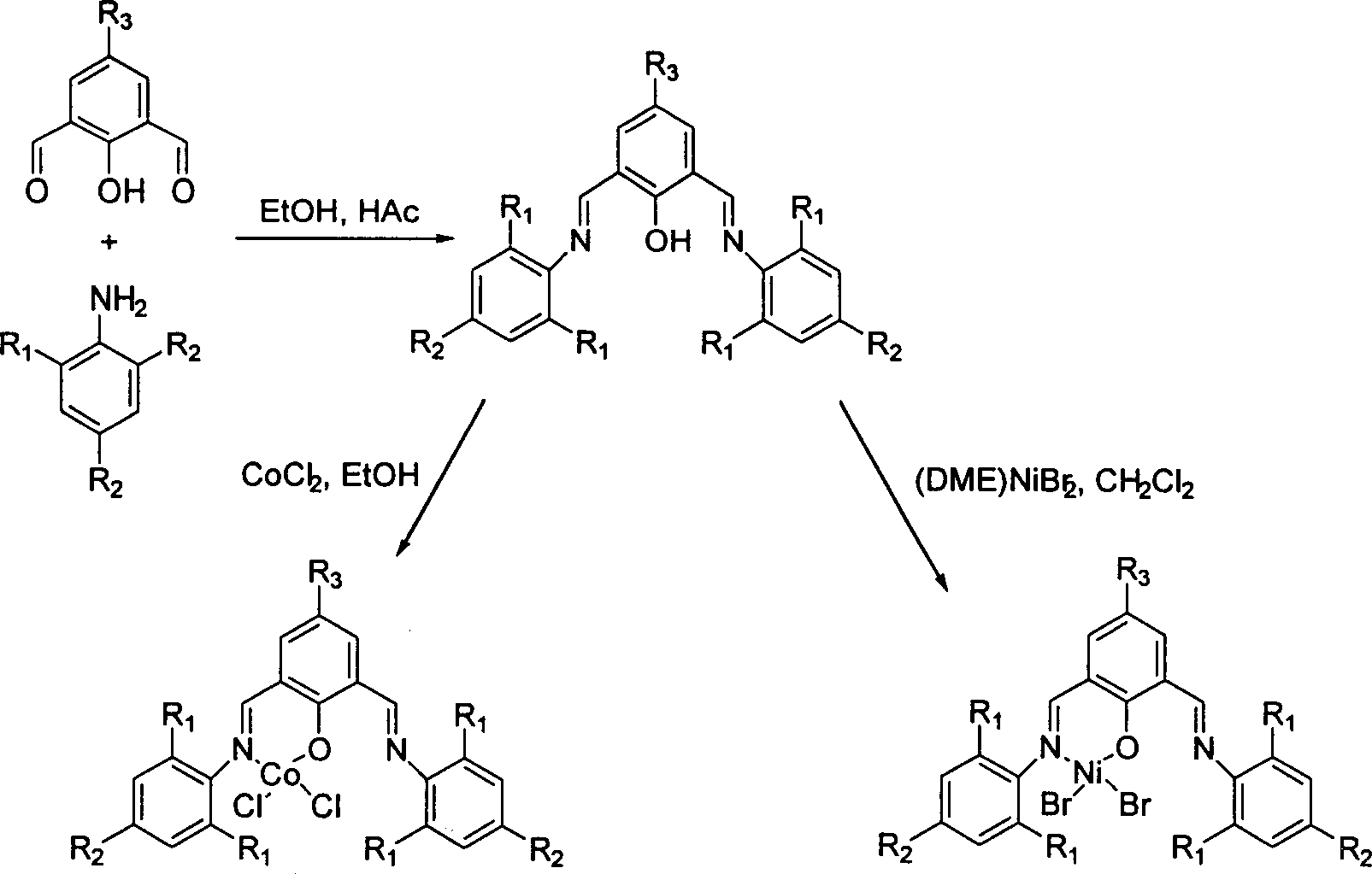
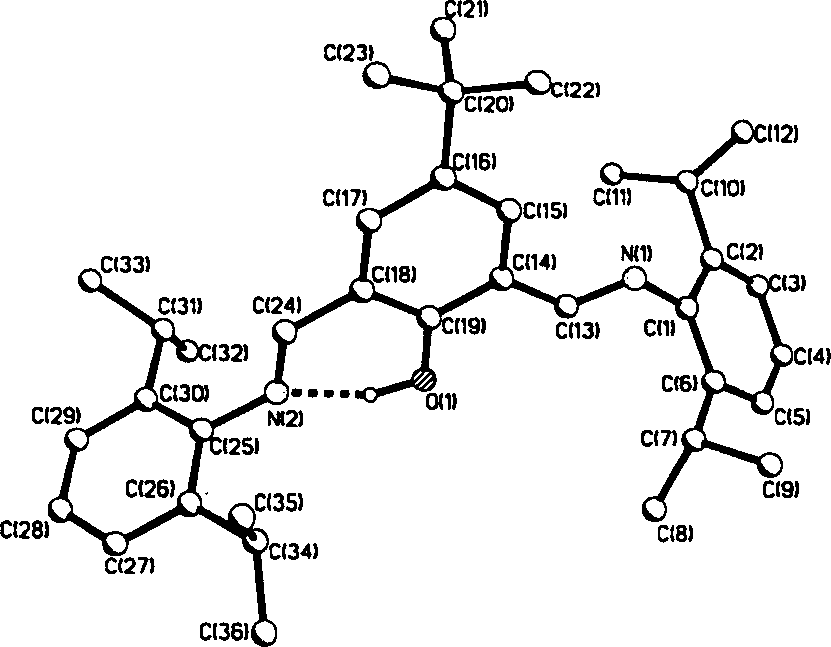
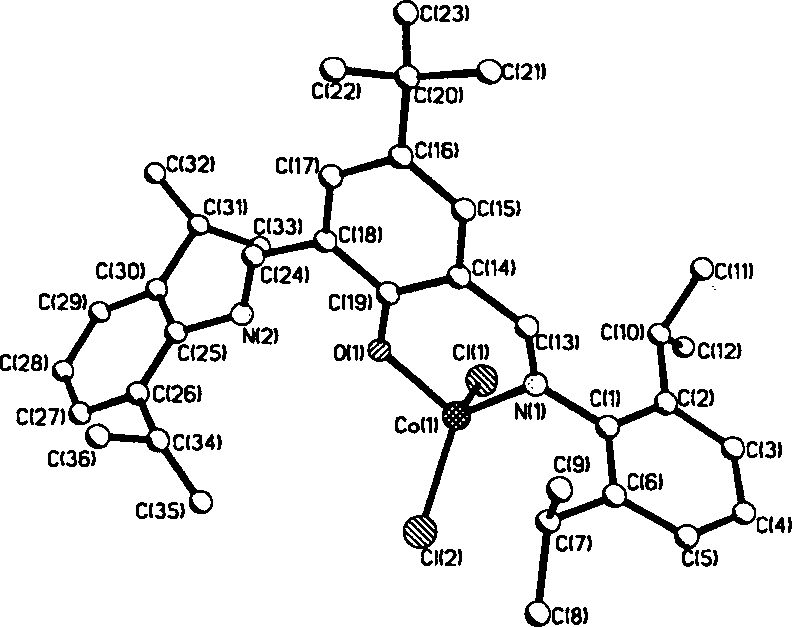
Ethylene oligomerization after-trasition metal coordination catalyst and its application
A late-transition metal and coordination catalyst technology, applied in organic chemistry, hydrocarbons, hydrocarbons, etc., can solve the problems of complex synthesis steps and preparation methods, and achieve wide distribution of catalytic activity, high catalytic activity, and easy purification Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Synthesis of ligand 2,6-bis(2,6-diisopropylphenylimino)-4-tert-butylphenol: under nitrogen protection, 4-tert-butyl-2,6-dimethyl Acylphenol (1.76g, 6mmol) was dissolved in absolute ethanol solution (25mL), then 3 drops of acetic acid were added, then the temperature was raised to 50°C, and 2,6-diisopropylaniline (2.16g , 13.2mmol) in absolute ethanol (15mL). After dripping, heat to reflux, continue to stir for 2 hours, cool to room temperature, evaporate most of the solvent under reduced pressure, and place the product mother liquor in the refrigerator overnight, 2.8 g of yellow crystals precipitate out, yield: 70%. m.p.192.5-193℃.IR(KBr): 3414(m), 3063(m), 2963, 2869(s), 1629, 1586(vs), 1540(s), 1460(s), 1360, 1313, 1282 , 1227, 1179(s), 819, 758(s); 1 H NMR (CDCl 3 ): δ1.27 (24H, d, J=6.8Hz), 1.49 (9H, s), 3.04-3.14 (4H, m), 7.24 (6H, m), 7.5 (1H, br), 8.37-8.80 ( 3H, br); EI-MS (m / z): 524 (M + , 7.8%), 509 (5.1%), 349 (23.6%), 348 (100%), 319 (5.5%); 176 (12.3%)...
Embodiment 2
[0026] The synthesis of ligand is the same as in Example 1
[0027] Preparation of 2,6-bis(2,6-diisopropylphenylimino)-4-tert-butylphenol nickel complex (2) catalyst:
[0028] Under nitrogen, to a 30 mL Schlenk tube was added 2,6-bis(2,6-diisopropylphenylimino)-4-tert-butylphenol (167 mg, 0.32 mmol), (DME)NiBr 2 (77mg, 0.25mmol), then inject freshly treated dichloromethane (10mL), stir at 25°C for 18 hours, distill off excess dichloromethane, add 5mL of anhydrous ether, filter the precipitated solid, and wash with ether (3×2 mL). Vacuum drying gave 124 mg of dark green solid powder, yield: 73%. m.p.238℃(dec.).IR(KBr): 3389(br), 2964(m), 1637(s), 1538(s), 1463(m), 1390(m), 1363, 1328, 1289, 1234 , 1177(m), 1060(m); FAB-MS(m / z): 663(M + -Br), 582 (M + -2Br), 525 (M + -NiBr 2 ); Anal.Calcd.for C 36 h 48 N 2 ONiBr 2 ·H 2 O: C, 56.80; H, 6.62; N, 3.68. Found: C, 56.80; H, 6.37; N, 3.52%.
[0029] Oligomerization of ethylene under normal pressure: put a stirring magnet ...
Embodiment 3
[0034] Synthesis of ligand 2,6-bis(2,6-dimethylphenylimino)-4-tert-butylphenol: Synthesis of ligand 2,6-bis(2,6-bis isopropylphenylimino)-4-tert-butylphenol method, to obtain yellow solid 2,6-two (2,6-dimethylphenylimino)-4-tert-butylphenol, yield Rate: 79%. m.p: 162.5-163°C. IR(KBr): 3368(Br), 2962(s), 1630, 1588(s), 1469(s), 1369(s), 1310, 1284, 1263, 1232, 1190(m), 1091, 1034, 1006 (m), 858, 765 (m); 1 H NMR (CDCl 3 ): δ1.44 (9H, s), 2.24 (12H, s), 6.95-7.14 (6H, m), 7.3-8.60 (4H, br); EI-MS (m / z): 412 (M+, 6.6 %), 411 (4.4%), 292 (100%), 291 (85.4%), 290 (8.3%), 277 (6.6%), 206 (5.1%), 132 (8.2%), 120 (6.9%) , 105 (10.1%); Anal.Calcd.for C 28 h 32 N 2 O: C, 81.51; H, 7.82; N, 6.79. Found: C, 81.22; H, 8.21; N, 6.34%.
[0035] Preparation of 2,6-bis(2,6-dimethylphenylimino)-4-tert-butylphenol cobalt complex (3) catalyst:
[0036] Using Example 1 to synthesize 2,6-bis(2,6-isopropylphenylimino)-4-tert-butylphenol CoCl 2 method using ligands 2,6-bis(2,6-dimethylphen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com