Derivative of micronomycine and its preparing process and medical application
A technology of micronomycin and derivatives, applied in the field of new aminoglycoside antibiotic derivatives, which can solve the problems of aminoglycoside antibiotics losing activity and hindering interaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
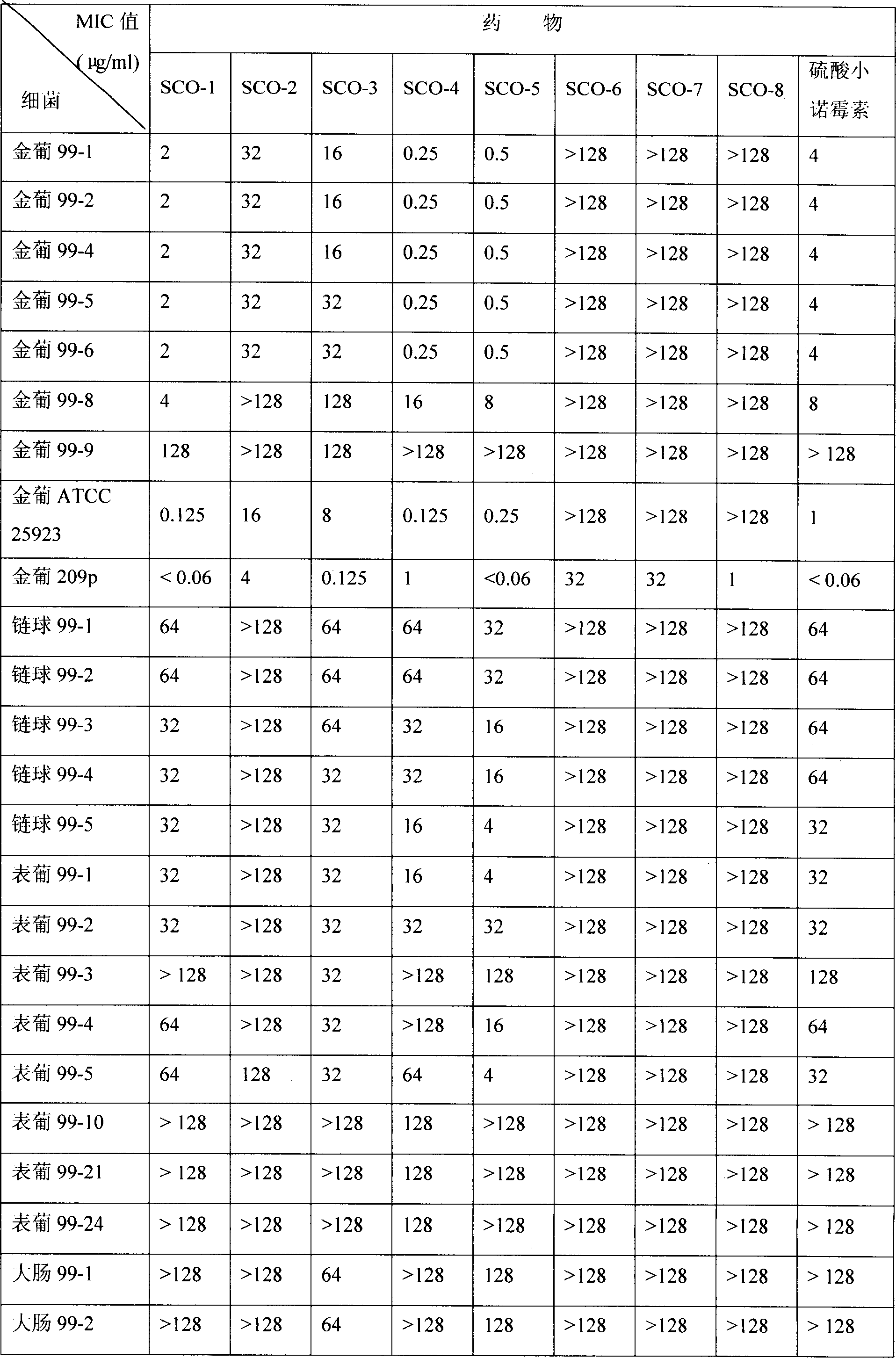
[0059] Embodiment 1: SCO-1 (C 1 -N-methyl-minornomycin) preparation
[0060] Feeding: Formaldehyde / THF 4.5ml
[0061] According to the above-mentioned general operation method A method, 2.9 g of SCO-1 was obtained from 14.1 g of triacetyl minonomycin. [α] D t 124.2°; elemental analysis: C: 52.83 H: 9.01 N: 14.67 (theoretical value: C: 52.72 H: 9.20 N: 14.60). IR (cm-1) 3361, 2936, 1452, 1337 MS (m / e) 477 364 360 314 160143 43 28 HNMR (D 2 O)δ(ppm)5.25(H-1′1H d)5.05(H-1″1H d)4.45(H-2′1H d J 1″-2″ =3.5HZ J 2″-3″ =11.0HZ)3.92(H-3″1H s)2.50(3″-N-CH 3 3HS)2.30(6'-NCH 33H S) 1.97 (4″-C-CH 3 3H S) 1.20 (1-NCH 3 3H S).
Embodiment 2
[0062] Embodiment 2: SCO-2 (C 1 -N-ethyl-micronomycin) preparation
[0063] Feeding: 4.5ml of acetaldehyde / tetrahydrofuran
[0064] According to the above-mentioned general operation method A, 2.5 g of SCO-2 was obtained from 14.2 g of triacetyl minonomycin. [α] D t 115.4°; elemental analysis: C: 53.70 H; 9.40 N: 14.20 (theoretical value: C: 53.77 H: 9.37 N: 14.30). IR (cm-1) 3361 2935 1453 1373 MS (m / e) 491 375 361 346314 289 233 160 143 115 100 43 HNMR (D 2 O)δ(ppm)5.27(H-1′1H d)5.00(H-1″1H d)4.45(H-2″1H d J 1″-2″ =3.5HZ J 2″-3″ =11.0HZ)3.92(H-3″1H s)2.48(3″-N-CH 3 3HS)2.35(6'-NCH 3 3H S) 1.95 (4″-C-CH 3 3H S) 1.30 (1-NCH 2 CH 3 2Hq)1.25(1 NCH 2 CH 3 3Ht).
Embodiment 3
[0065] Embodiment 3: SCO-3 (C 1 -N-isobutyl-micronomycin) preparation
[0066] Feeding: 4ml of isobutyraldehyde / tetrahydrofuran
[0067] According to the above-mentioned general operation method A method, 1.9 g of SCO-3 was obtained from 14.1 g of triacetyl minonomycin. [α] D t 119°; elemental analysis: C: 55.40 H: 9.51 N: 13.49 (theoretical value: C: 55.50 H: 9.63 N: 13.48). IR (cm-1) 3360 2935 1452 1390 1372 MS (m / e) 519 375 347 314 272 233 205 160 143 73 57 44 HNMR (D 2 O)δ(ppm)5.25(H-1′1H d)5.01(H-1″1H d)4.46(H-2″1H d J 1″-2″ =3.5HZ J 2″-3″ =11.0HZ)2.45(3″-NCH 3 3HS)2.32(6'-NCH 3 3H S) 1.96 (4″-C-CH 3 3H S) 1.20 (1-NCH 2 -CH(CH 3 ) 2 2H d)0.90(1-NCH 2 CH(CH 3 ) 2 6H d).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com