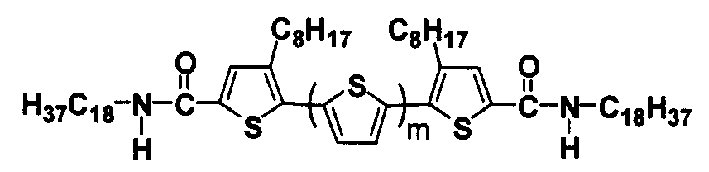
Oligothiophene derivative, its preparation method and application
A technology of oligothiophene and derivatives, applied in chemical instruments and methods, color-changing fluorescent materials, organic chemistry, etc., can solve problems such as difficult encapsulation, and achieve the effect of overcoming difficult encapsulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] preparation
[0020] The first step is at -78℃, N 2 Under protection, the THF solution of DOc3T was added into the THF solution of LDA with a syringe, at this time, the solution turned red, and the reaction was stirred for 1 hour;
[0021] In the second step, dry ice (CO 2 ), at this time, the solution turns yellow, and at -78°C, N 2 Under protection, continue to react for 5 hours, then warm up to room temperature, continue to react for 3 hours;
[0022] In the third step, dilute hydrochloric acid was added to the reaction solution, solid matter appeared, and the reaction was continued for 8 hours, THF and water were removed by filtration, washed with water, and dried to obtain a yellow powdery product;
[0023] In the fourth step, the product is added to thionyl chloride (SOCl 2 ) in 1,2-dichloroethane solution until completely dissolved, reflux reaction for 5 hours, and remove the solvent;
[0024] The fifth step is to mix the product with stearylamine at 0°C und...
Embodiment 2
[0031] preparation
[0032] The first step is at -78℃, N 2 Under the protection of expansion, add the THF solution of DOc4T into the THF solution of LDA with a syringe. At this time, the solution turns red, and the reaction is stirred for 3 hours;
[0033] In the second step, dry ice (CO 2 ), at this time, the solution turns yellow, and at -78°C, N 2 Under protection, continue to react for 3 hours, then gradually warming up to room temperature, continue to react for 5 hours;
[0034] The third step is to add dilute hydrochloric acid to the reaction solution, solid matter appears, continue to react for 5 hours, remove THF and water by filtration, wash with water several times, and dry to obtain a yellow powder product;
[0035] In the fourth step, the product is added to thionyl chloride (SOCl 2 ) in 1,2-dichloroethane solution until completely dissolved, reflux for 8 hours, and remove the solvent;
[0036] The fifth step is to mix the product with stearylamine at 0°C unde...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com