Polythiophene derivative with side chain containing naphthalene groups and preparing method and application of polythiophene derivative
A kind of technology of polythiophene derivative, methylthiophene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
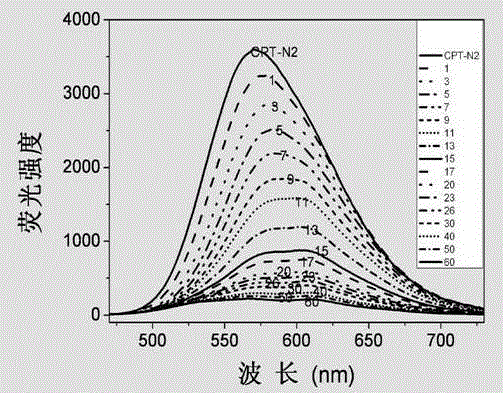
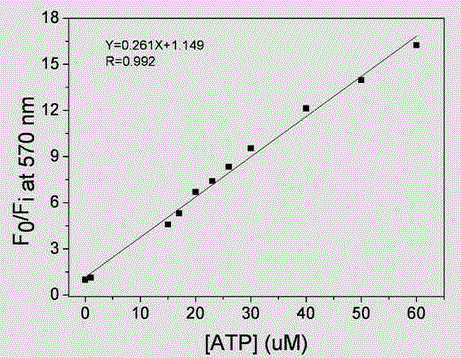
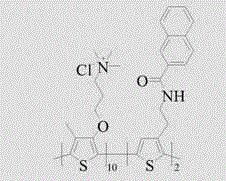
[0030] A polythiophene derivative containing a naphthalene group in the side chain, which is a combination of 4-methyl-3-(4'-trimethylammonium chloride propoxy)-thiophene and 3-(naphthalene amidoethyl)thiophene Copolymer, its structural formula is:
[0031]
[0032] Its preparation method, the steps are as follows:
[0033] 1) Synthesis of 3-methoxy-4-methylthiophene
[0034] Add 27.6mmol of 3-bromo-4-methylthiophene and 17.4mol of cuprous bromide to 18mL of 28wt% sodium methoxide-methanol solution, then add 7mL of solvent nitrogen methylpyrrolidone to obtain a mixed solution, and reflux under nitrogen protection After 3 days, after cooling to room temperature, the solid was filtered off, the filtrate was extracted with ether, and the obtained organic phase was washed with anhydrous MgSO 4 After drying, the solvent was evaporated to dryness, and the crude product was purified by column chromatography using n-hexane as the eluent to obtain 13.25 g of 3-methoxy-4-methylthio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com