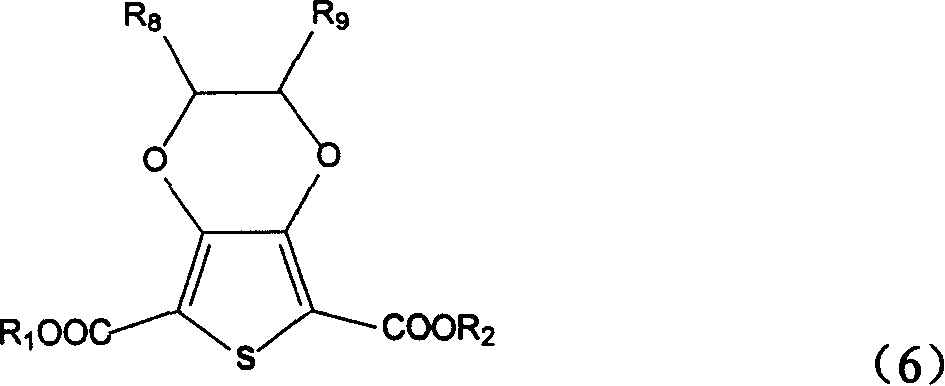
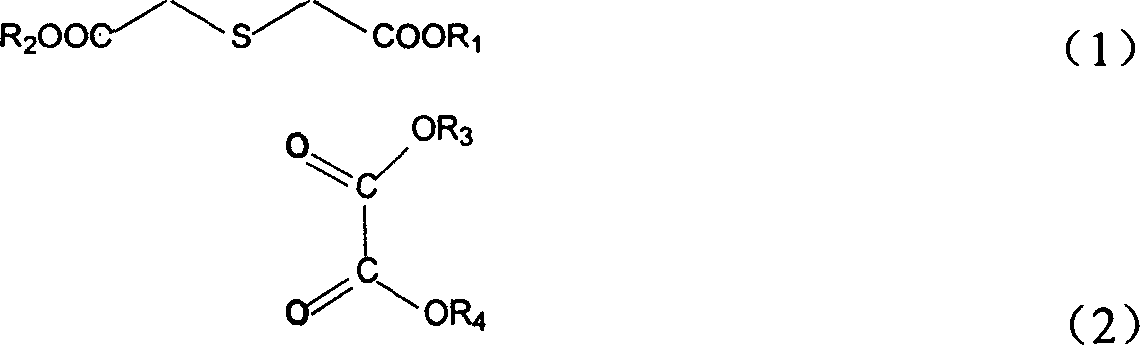
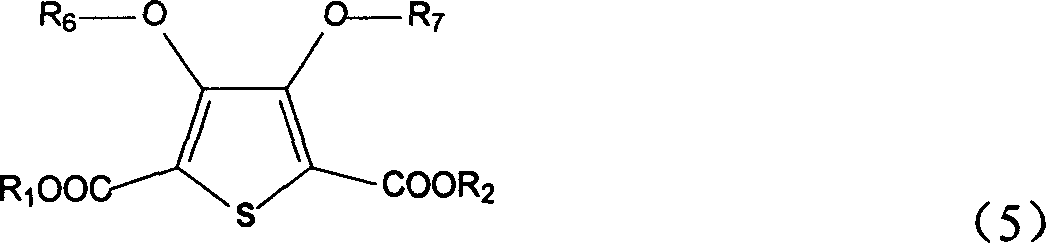Position fixable polymerized precursor of polythiazole monomer and its synthesis
A synthesis method and technology of polythiophene are applied in the field of preparing precursors of thiophene polymer monomers, which can solve the problems of long reaction period, low yield of final product compounds, and difficulty in obtaining ideal yields, and shorten the reaction time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Add 5.0 g (0.028 mol) of dimethyl thiodiglycolate, 8 g (0.055 mol) of diethyl oxalate, and 1.5 g of potassium carbonate into reactor 1 containing 50 ml of anhydrous methanol, and mix well. Mix 7.5g (0.14mol) of sodium methoxide with 65ml of anhydrous methanol and pour it into a three-neck flask 2 with block anhydrous copper sulfate built in, cool down to make the reaction solution 0-2°C, and put the The ester mixture was slowly added dropwise into flask 2, and after the dropwise addition was completed, it was heated to reflux for a reaction time of 1.5 hours. Suction filtration, repeated (3-5 times) washing with anhydrous methanol, vacuum drying to obtain the reaction product 2,5-dicarboxylate-3,4-diphenol sodium thiophene 6.95g, yield 90%, HPLC : 92.0%.
[0053] Elemental analysis value of the product: C, 35.3%; H, 2.40%.
[0054] Infrared Spectrum Absorption Peak: The absorption peak is 1725cm -1 , The absorption peak is 1532cm -1 , 2955cm -1 It is the absorp...
Embodiment 2
[0057] 6.4g (0.031mol) diethyl thiodiglycolate, and 15 grams (0.103mol) diethyl oxalate, 1.6g potassium carbonate were added together in the glass reaction bottle a that 50ml absolute ethanol was housed, and 20g ( 0.082mol) of sodium trityl was mixed with 20ml of ethanol and then added to another reaction bottle b with a built-in desiccant of block CaO, and the temperature was controlled at -2°C-0°C. Slowly add the ester mixture into the reaction bottle a dropwise. After the dropwise addition, the temperature is raised to 80° C., and the reaction time is 1.5-2.0 h. After suction filtration, washing with water three times, and vacuum drying, 7.4 g of the product 2,5-dicarboxylate-3,4-diolthiophene was obtained, with a yield of 89%. HPLC: 93.0% purity.
[0058] Elemental analysis results: C, 46.1%; H, 4.7%; O, 37.0%; S, 12.4%
[0059] The infrared spectrum analysis result of product is as follows:
[0060] The absorption peak is 1728cm -1 , the absorption peak of the carbo...
Embodiment 3
[0068] Embodiment 3 (mixed solvent)
[0069] 8.0g of diethyl thiodiglycolate, together with 15 grams (0.103mol) of diethyl oxalate, and 2.0g of sodium carbonate were added to a glass reaction bottle a containing 50ml of anhydrous methanol, and 20g of sodium ethylate was mixed with 75ml of After mixing absolute ethanol, add it to the built-in block anhydrous CaSO 4In another reaction bottle b of the desiccant, the temperature is controlled at 0°C-2°C. Slowly add the ester mixture into the reaction flask b dropwise. After the dropwise addition, the temperature is raised to 70° C., and the reaction time is 2.5-3.0 h. After suction filtration, washing with water three times, and vacuum drying, the product 2,5-ethyl dicarboxylate-3,4-diolthiophene was obtained with a yield of 88.5%. HPLC: 93.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com