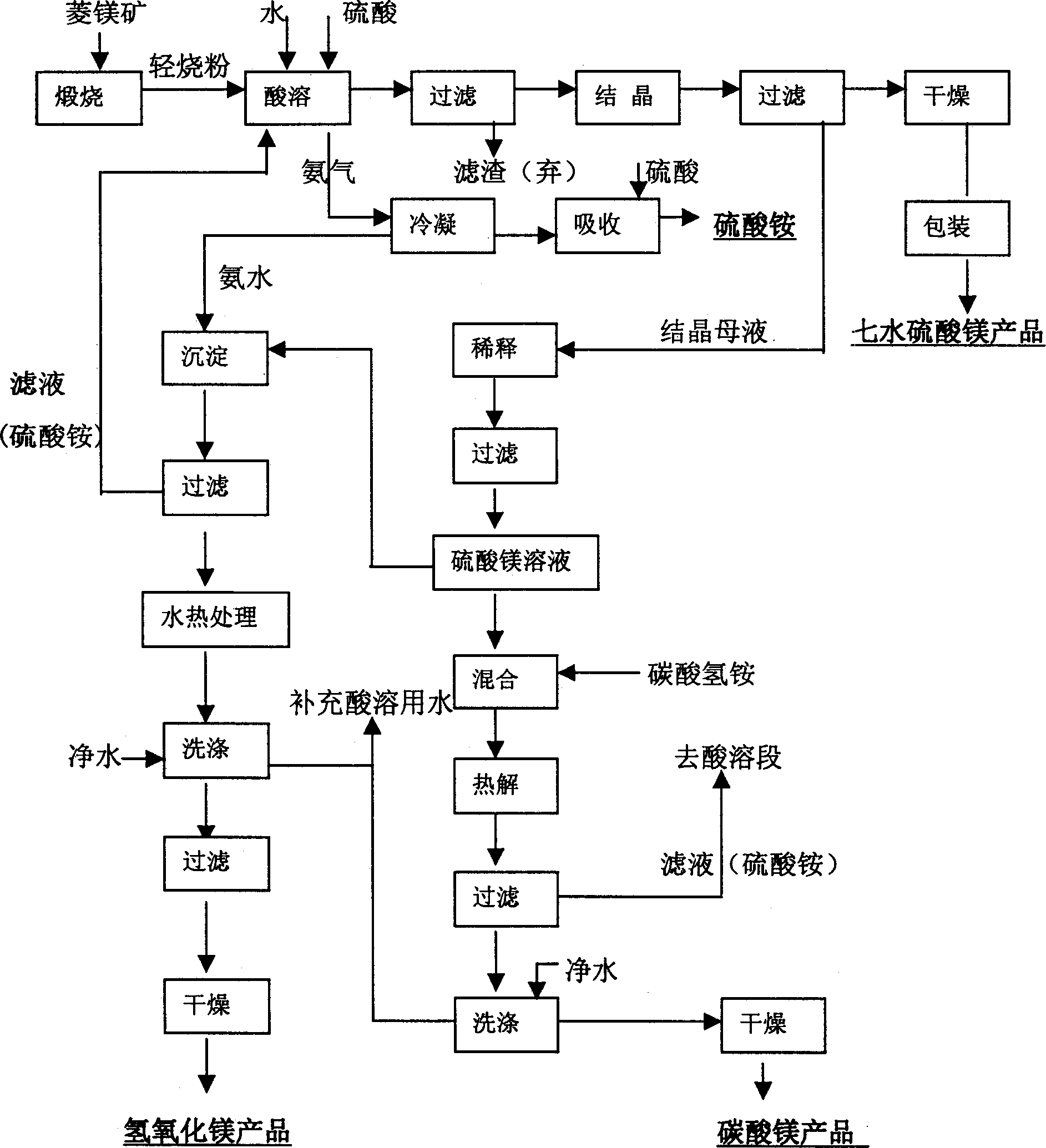
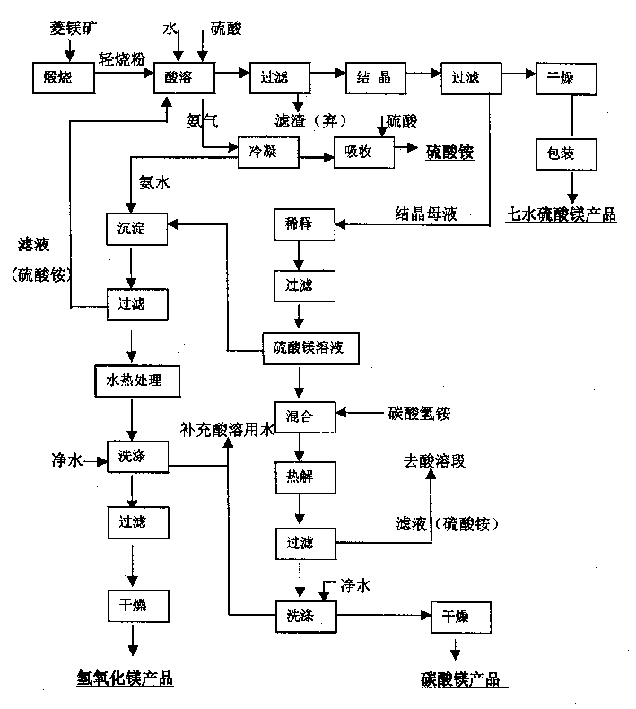
Method for integrated production of magnesium sulfate, magnesium carbonate and magnesium hydroxide from magnesite
A magnesium hydroxide and joint production technology, applied in the direction of magnesium hydroxide, magnesium carbonate, magnesium sulfate, etc., can solve the problems of unfavorable environmental protection requirements, high production costs, and inability to fully utilize by-products, so as to achieve environmental protection and reduce production cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Use a meter to measure 1500 liters of 2.0mol / L ammonium sulfate solution and add it to a 2 cubic meter reaction kettle. Add 270 kg of -120 mesh light-burned magnesium oxide powder under stirring conditions. After sealing the system, start stirring and slowly add 98% Industrial concentrated sulfuric acid, ensure that the temperature reaches above 90°C within 20 minutes, so that the ammonia gas is evaporated, and the ammonia gas evaporated within the first 30 minutes is condensed by the condenser to obtain ammonia water with a higher concentration (the concentration is 18%). into the storage tank as the raw material for the next step of magnesium hydroxide precipitation. After 30 minutes, the concentration of ammonia water is low, and concentrated sulfuric acid is added for neutralization to prepare ammonium sulfate by-product.
[0032] Reduce the acidity of the reaction system to pH 5.5-6.5 within 2 hours, discharge the material into the transition tank, pump it into the...
Embodiment 2
[0041] Add 1500 liters of ammonium sulfate solution that 2.0mol / L magnesium hydroxide and magnesium carbonate precipitation process obtains with meter to add in the reactor of 2 cubic meters, add 300 kilograms of -120 order light-burned magnesium oxide powder under stirring condition, will After the system is sealed, start stirring and slowly add 98% industrial concentrated sulfuric acid to ensure that the temperature reaches above 90°C within 20 minutes to evaporate the ammonia gas. The ammonia gas evaporated within the first 25 minutes is condensed by the condenser to obtain a higher concentration The ammoniacal liquor (its concentration is 20%) is put into storage tank as the raw material of next step magnesium hydroxide precipitation. After 25 minutes, the concentration of ammonia water is low, and concentrated sulfuric acid is added to neutralize the by-product of ammonium sulfate.
[0042] Reduce the acidity of the reaction system to pH 6.0-6.5 within 1.5 hours, discharg...
Embodiment 3
[0046] Use a meter to measure 1500 liters of ammonium sulfate solution produced by magnesium carbonate and magnesium hydroxide precipitation and add it to a 2 cubic meter reaction kettle. Add 400 kilograms of -120 mesh light-burned magnesium oxide powder under stirring conditions. After sealing the system, start Stir and slowly add 98% industrial concentrated sulfuric acid to ensure that the temperature reaches above 90°C within 20 minutes to evaporate the ammonia gas. The ammonia gas evaporated within the first 20 minutes is condensed by the condenser to obtain ammonia water with a higher concentration (the concentration 21%) is put into storage tank as the raw material of next step magnesium hydroxide precipitation. After 20 minutes, the concentration of ammonia water is low, and concentrated sulfuric acid is added to neutralize the by-product of ammonium sulfate.
[0047] Reduce the acidity of the reaction system to pH 6.0-6.5 within 1.5 hours, discharge the material into t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com