Precursors of silk-like materials, silk-like materials and processes for producing both
A precursor and spinning technology, applied in the direction of botany equipment and methods, biochemical equipment and methods, chemical instruments and methods, etc., can solve the problem of not being able to clearly explain the mechanism of generating silk threads, not knowing the correct mechanism of silk threads, and being unable to Synthetic threads etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] The preparation method of the silk-like material precursor of the present invention is described in detail below. During the preparation of the precursor of the present invention, the kind and amount of other amino acids used together with glycine are first determined. Only one kind is preferably used, but a plurality of kinds may be used. In addition, the amount of Y used is determined according to the desired degree of water solubility. The amount of Y used is preferably less than or equal to 20% (mol) of all amino acids. When the amount of Y is too large, the stability of the obtained precursor becomes poor, thereby making fibrillation difficult. In addition, it is necessary to introduce Y in the main chain. Moreover, by A 1 represents each third A of the alanine sequence 1 The hardness of the copolymer can be adjusted by introducing serine into it.
[0019] The precursor of the present invention can be synthesized by a solid-phase peptide synthesis method, or ...
Embodiment Embodiment 1
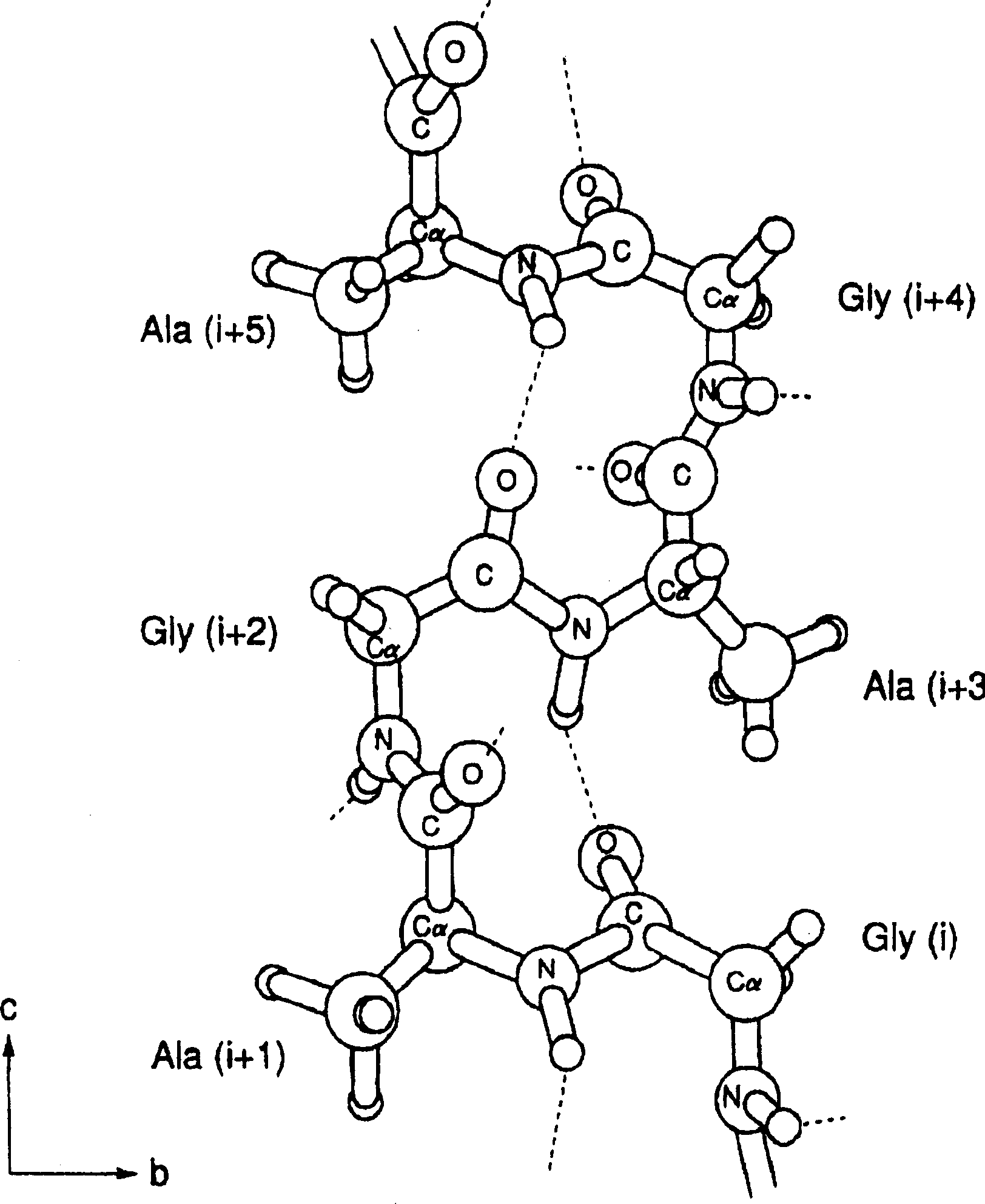
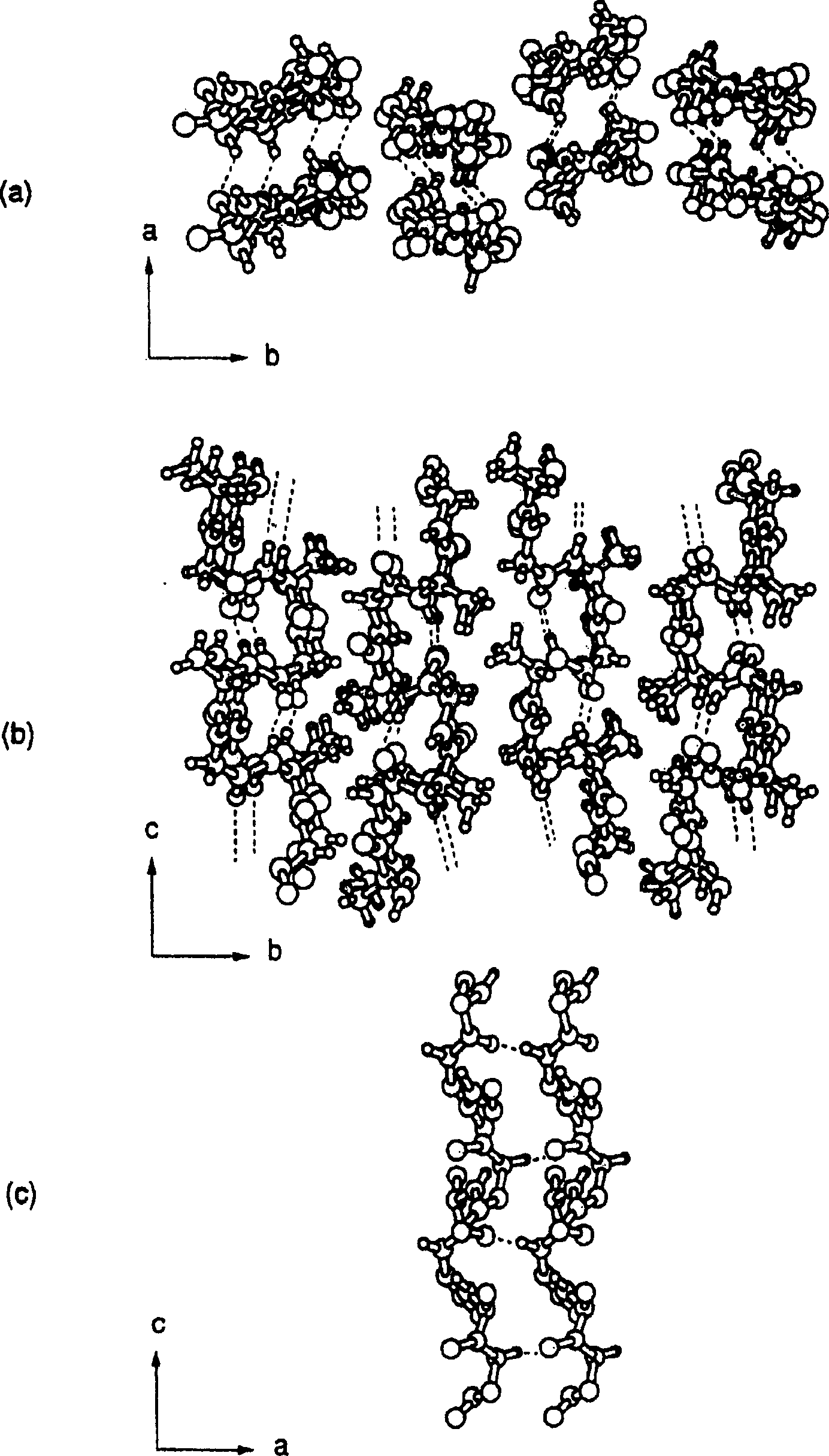
[0033] A comparative examination of the structure and structural changes of tyrosine residues in silk was performed. By giving silkworm larvae 50mg 13 C-labeled L-tyrosine and the silk L-tyrosine residues of Bombyx mori silk 13 C isotope labeling, followed by solid high resolution 13 C NMR determination. The results demonstrated that in liquid silk and films dried from it, L-tyrosine residues adopt a random coil structure, but in silk fibers obtained from silkworm cocoons, they adopt a Silk type II structure. Next, oriented solid-state NMR measurements were performed on fibers obtained by dissolving and spinning the above-mentioned membranes in hexafluoroisopropanol (HFIP) and on silk threads obtained from silkworm cocoons, and in all cases, it was confirmed that the L-tyrosine residue adopts Silk II type structure, oriented in each molecular chain at the same time. In addition, it was confirmed by solid-state NMR that alanine and glycine residues, the main components of s...
Embodiment 2
[0034] The following compounds I, II, III and IV, which are representative chain portions of the repeat region of Bombyx mori silk, were synthesized using a solid-phase peptide synthesizer, and their water solubility was also determined. The results confirmed that I was completely insoluble in water and II, III and IV were very soluble. Through these experiments, it was confirmed that tyrosine residues are essential for the water solubility of silk. Synthesized compound: G(AG) 2 SG(AG) 2 IG(AG) 2 YG(AG) 2 IIGAGVGYG (AG) 2 IIITGFDSESSWAYEYGSYGGNAVYPGFGSSGT IV Among them, G is glycine, A is alanine, Y is tyrosine, V is valine, T is threonine, F is phenylalanine, D is aspartic acid, E is glutamic acid Acid, W is tryptophan, N is asparagine, P is proline, all of these amino acids are L-form except glycine. Example 3:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com