Dosing form for reagents, use of said dosing form in organic chemical synthesis and production of said dosing form
A technology of reagents and dosage forms, applied in the quantitative field of solid reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
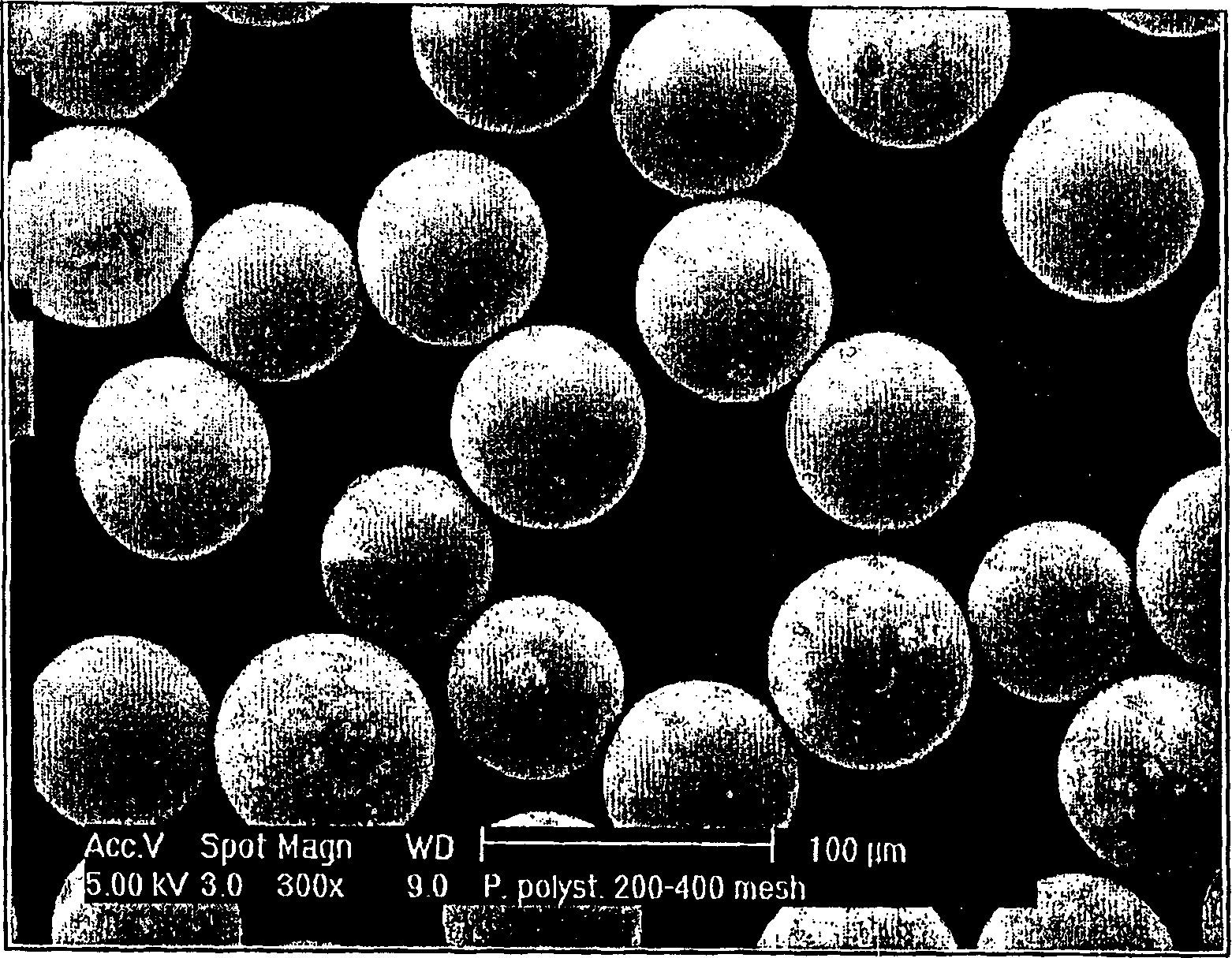
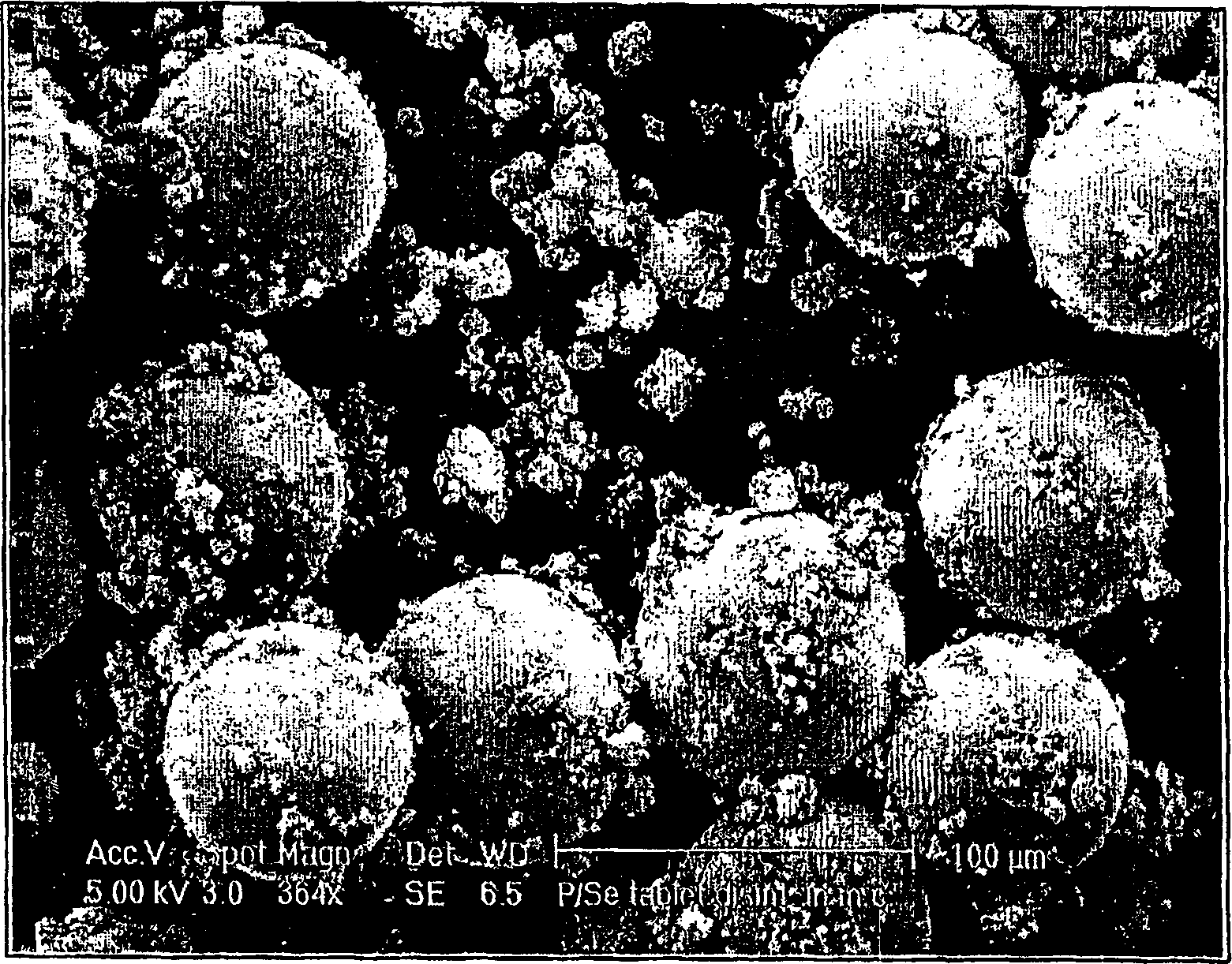
Image
Examples
Embodiment 1
[0119] Example 1. Mitsunobu reaction using tablets comprising polystyrene, di-tert-butylazodicarboxylate and diphenylphosphorylpolystyrene with tablets of isocyanatomethylpolystyrene
[0120] The evaluation results are summarized in Table 3. A detailed description of the steps described follows:
[0121]2-(2-Phenylthio-ethyl)-2H-naphtho[1,8-cd]isothiazole 1,1-dioxide (entry 1). Tablets containing a total of 0.22 mmol di-tert-butylazodicarboxylate and 0.29 mmol resin-bound diphenylphosphine were added with 2H-naphtho[1,8-cd]isothiazole 1,1- Solution of dioxide (21.0 mg, 0.10 mmol) and 2-phenylthio-ethanol (29.9 mg, 0.20 mmol) in THF (3 mL). After stirring for 16 h, THF (2 mL) and a tablet containing isocyanatomethylpolystyrene (150 mg, 0.15 mmol) were added. The mixture was stirred at 60 °C for 2 h. The resin was filtered and washed with dichloromethane (1 x 1 mL), methanol (1 x 1 mL) and dichloromethane (1 x 2 mL). Trifluoroacetic acid (0.4 mL) was added to the combined f...
Embodiment 2
[0129] Example 2. Acylation reaction using tablets comprising polystyrene, tetrabromomethane and diphenylphosphorylpolystyrene with tablets of isocyanatomethylpolystyrene
[0130] The evaluation results are summarized in Table 4. A detailed description of the steps described follows:
[0131] 4-Morpholinocarbonylferrocene (entry 6). To a tablet containing 0.11 mmol tetrabromomethane and 0.15 mmol resin-bound diphenylphosphine was added ferrocenecarboxylic acid (23.3 mg, 0.10 mmol), morpholine (10.7 mg, 0.12 mmol) and triethylamine at 0 °C (22.6 mg, 0.22 mmol) in dry THF (1.5 mL). After stirring at room temperature for 16 h, THF (2 mL) and a tablet (150 mg) containing isocyanatomethylpolystyrene (0.15 mmol) were added. The mixture was stirred at 60 °C for 2 h. The resin was filtered and washed with dichloromethane (1 x 1 mL), methanol (1 x 1 mL) and dichloromethane (1 x 2 mL). The solvent was evaporated in vacuo and the residue was purified by solid phase extraction (hepta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com