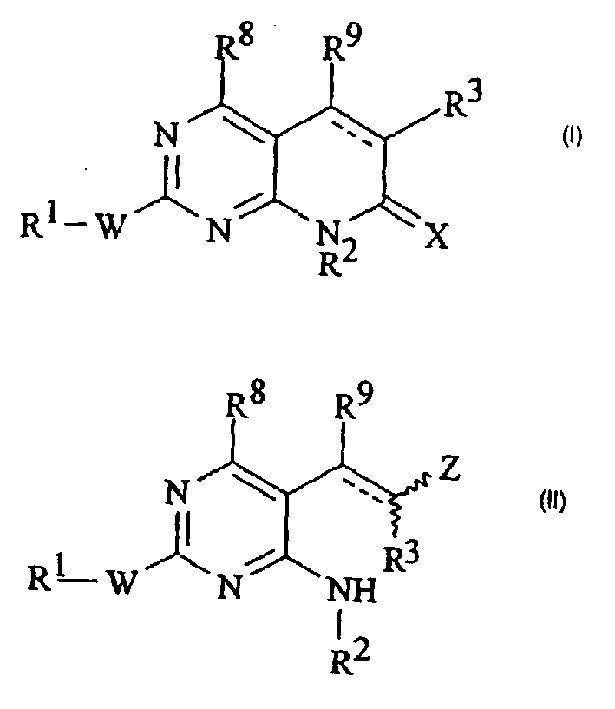
Pyridopyrimidinone derivatives for treatment of neurodegenerative disease
A neurodegenerative disease, pyridine technology, applied in the field of novel compounds, can solve the problem of marginal effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0589] 4-Ethylamino-2-methylthio-pyrimidine-5-carboxylic acid ethyl ester
[0590]To a room temperature solution of ethyl 4-chloro-2-methylthiopyrimidine-5-carboxylate (10.00 g, 43.10 mmol) in 150 mL of THF was added triethylamine (18.5 mL, 133 mmol) followed by 9 mL of 70% ethylamine aqueous solution. The solution was stirred for 30 minutes, then concentrated in vacuo, partitioned between chloroform and saturated aqueous sodium bicarbonate. The organic layer was dried over magnesium sulfate, filtered and concentrated to afford 9.32 g (90%) of ethyl 4-ethylamino-2-methylthio-pyrimidine-5-carboxylate as an oil.
[0591] Analytical calculated value C 10 h 15 N 3 o 2 S: C, 49.77; H, 6.27; N, 17.41.
[0592] Found values: C, 49.77; H, 6.24; N, 17.30.
Embodiment 2
[0594] (4-Ethylamino-2-methylthio-pyrimidin-5-yl)-methanol
[0595] A solution of ethyl 4-ethylamino-2-methylthio-pyrimidine-5-carboxylate (8.93 g, 37.1 mmol) in 100 mL THF was added dropwise to lithium aluminum hydride (2.30 g, 60.5 mmol) in 100 mL THF at room temperature in suspension. After 10 minutes, the reaction was carefully quenched with 4.5 mL of water, 4.5 mL of 15% NaOH and another 16 mL of water, and the mixture was stirred for 1.5 hours. The white precipitate was removed by filtration and washed with ethyl acetate. The filtrate was concentrated in vacuo and 1:1 hexane:ethyl acetate was added. The solid was collected to give 6.77 g (92%) of (4-ethylamino-2-methylthio-pyrimidin-5-yl)-methanol, mp 152-156°C.
[0596] Analytical calculated value C 8 h 13 N 3 OS: C, 48.22; H, 6.58; N, 21.09.
[0597] Found values: C, 48.14; H, 6.61; N, 20.85.
Embodiment 3
[0599] 4-Ethylamino-2-methylthio-pyrimidine-5-carbaldehyde
[0600] To a solution of (4-ethylamino-2-methylthio-pyrimidin-5-yl)-methanol (6.44 g, 32.4 mmol) in 600 mL of chloroform was added manganese oxide (21.0 g, 241 mmol). The suspension was stirred at room temperature for 2 hours and a further 5.5 g of manganese oxide were added. Stirring was continued for 4.5 hours. The mixture was filtered through celite, washing with chloroform. The filtrate was concentrated in vacuo to afford 6.25 g (97%) of 4-ethylamino-2-methylthio-pyrimidine-5-carbaldehyde, mp 58-61°C.
[0601] Analytical calculated value C 8 h 11 N 3 OS: C, 48.71; H, 5.62; N, 21.30.
[0602] Found values: C, 48.62; H, 5.60; N, 21.28.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com