Hepatocyte lineage cells derived from pluripotent stem cells
A technology of pluripotent stem cells and human pluripotent stem cells, applied in the field of directed differentiation, can solve the problems of mouse embryonic cells not responding to the same culture conditions and pluripotent cells being fragile
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0064] Preparation of primate pluripotent stem cells (pPS)
[0065] Embryonic stem cells can be isolated from fibroblasts of members of the primate species (Thomson et al., Proc. Natl. Acad. Sci. USA 92:7844, 1995). Human embryonic stem cells (hES) can be prepared from human fibroblasts using the techniques described by Thomson et al.
[0066] To obtain human fibroblasts, embryos preimplanted in vivo or in vitro fertilized (IVF) can be used, or single-cell human embryos can be expanded to the blastocyst stage (Bongso et al., Hum Reprod 4:706, 1989). Briefly, human embryos were grown to the blastocyst stage in G1.2 and G2.2 media (Gardner et al., Fertil. Steril. 69:84, 1998). Developed blastocysts can be selected for isolation of ES cells. The zona pellucida was removed from blastocysts by brief exposure to pronase (Sigma). The inner cell mass was separated by immunosurgery, in which blastocysts were exposed to 1:50 diluted rabbit anti-human splenocyte antiserum for 30 minut...
Embodiment 1
[0187] Example 1: Differentiation of human embryonic stem cells with n-butyrate
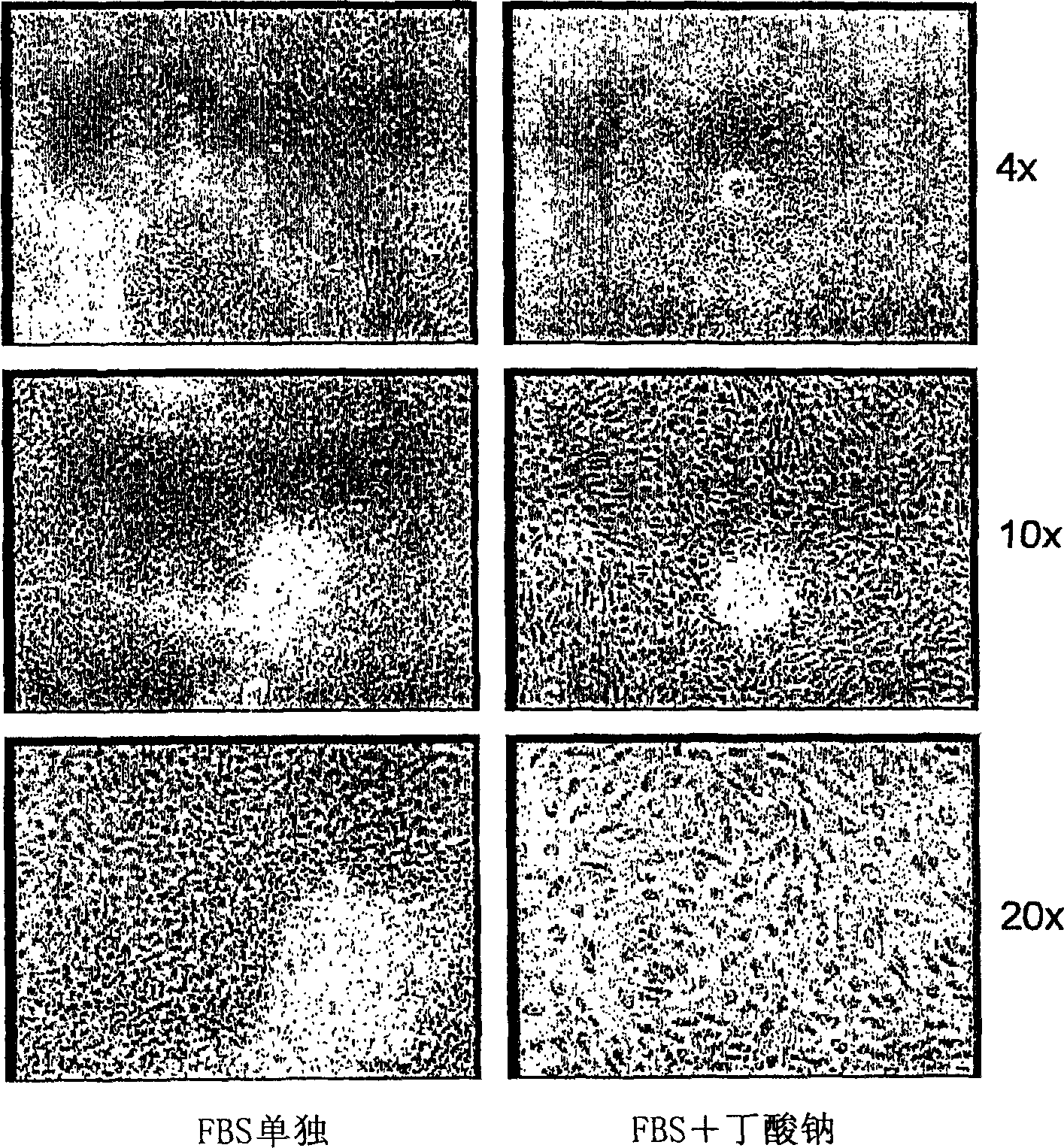
[0188] Embryoid bodies (EBs) were prepared as described in the previous section. After 5 days of suspension culture, they were harvested and plated on growth factor-reduced Matrigel(R) coated plates, and culture tank slides (Nunc). The following three conditions were used in parallel:
[0189] A culture medium containing 20% fetal bovine serum (FBS);
[0190] - Medium containing 20% FBS and 5 mM sodium butyrate (Sigma);
[0191] • Contains 20% FBS, 0.5% DMSO (ATCC), 4 μm dexamethasone (Sigma), 150 ng / ml insulin, 10 ng / ml EGF, 600 nM glucagon (Sigma).
[0192] In each case, medium was changed daily and cells were fixed for immunocytochemistry on day 4 after plating.
[0193] 1 day after plating, EBs plated alone in 20% FBS appeared healthy, they were almost all attached to the plate and appeared to be proliferating. After a few days, only the cells in FBS survived well and differentiated ...
Embodiment 2
[0199] Example 2: Markers Expressed by Differentiated Cells
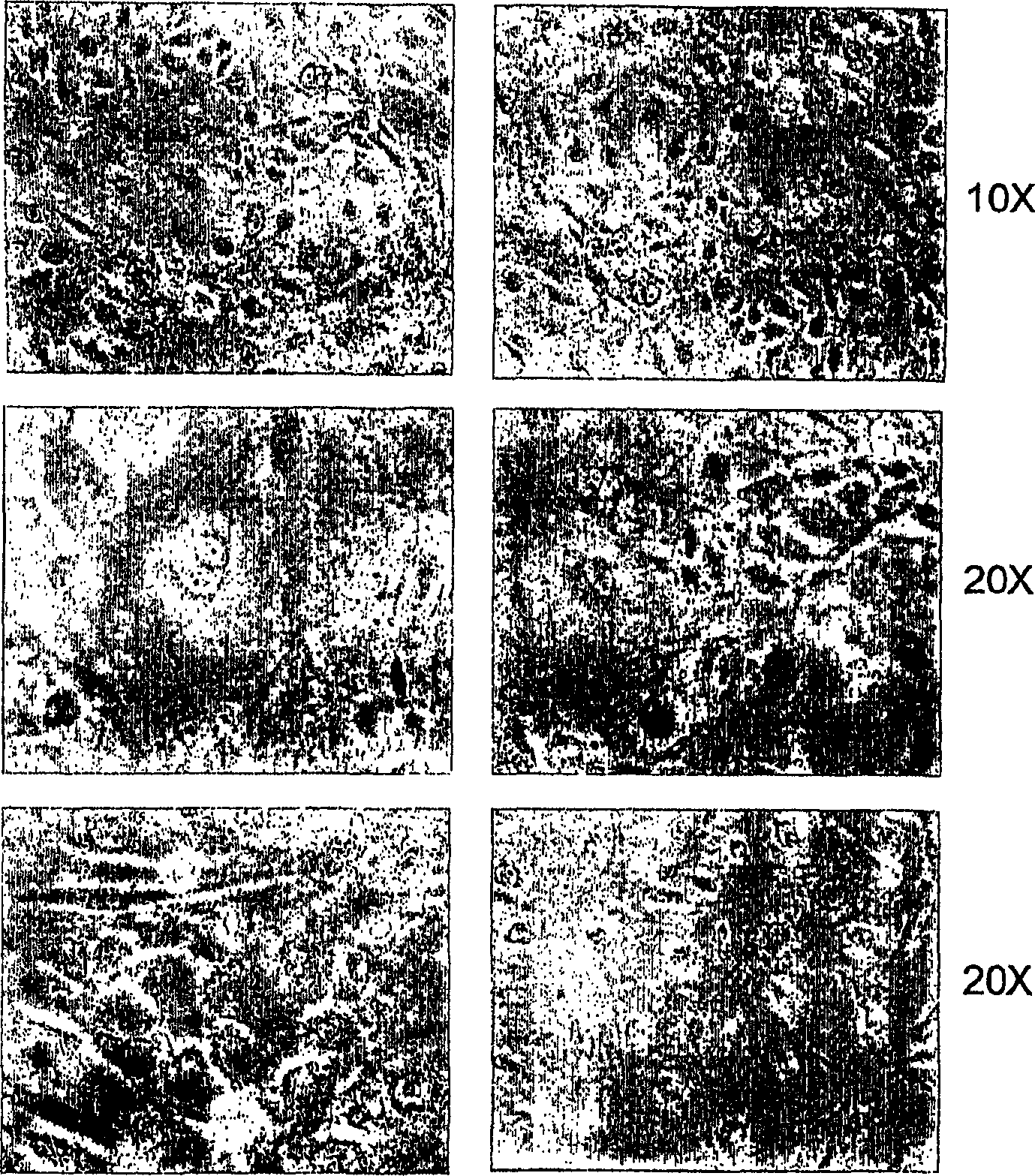
[0200] After 4 or 5 days of suspension culture, the embryoid bodies derived from hES cells were collected and plated on Matrigel®-coated 6-well plates (for RNA extraction) and culture tank slides (for immunocytochemistry) containing 20 % FBS and 5 mM sodium n-butyrate in the medium. Change the medium every day or every other day. Many cells die on day 1, then fewer cells die in the following days.
[0201] figure 2 Morphology of differentiated cells after 6 days of culture with n-butyrate is shown. The same culture shows 6 different fields (1Ox on top, 2Ox in other columns). The cells are remarkably homogeneous, displaying the large polygonal surface and binucleated center characteristic of mature hepatocytes.
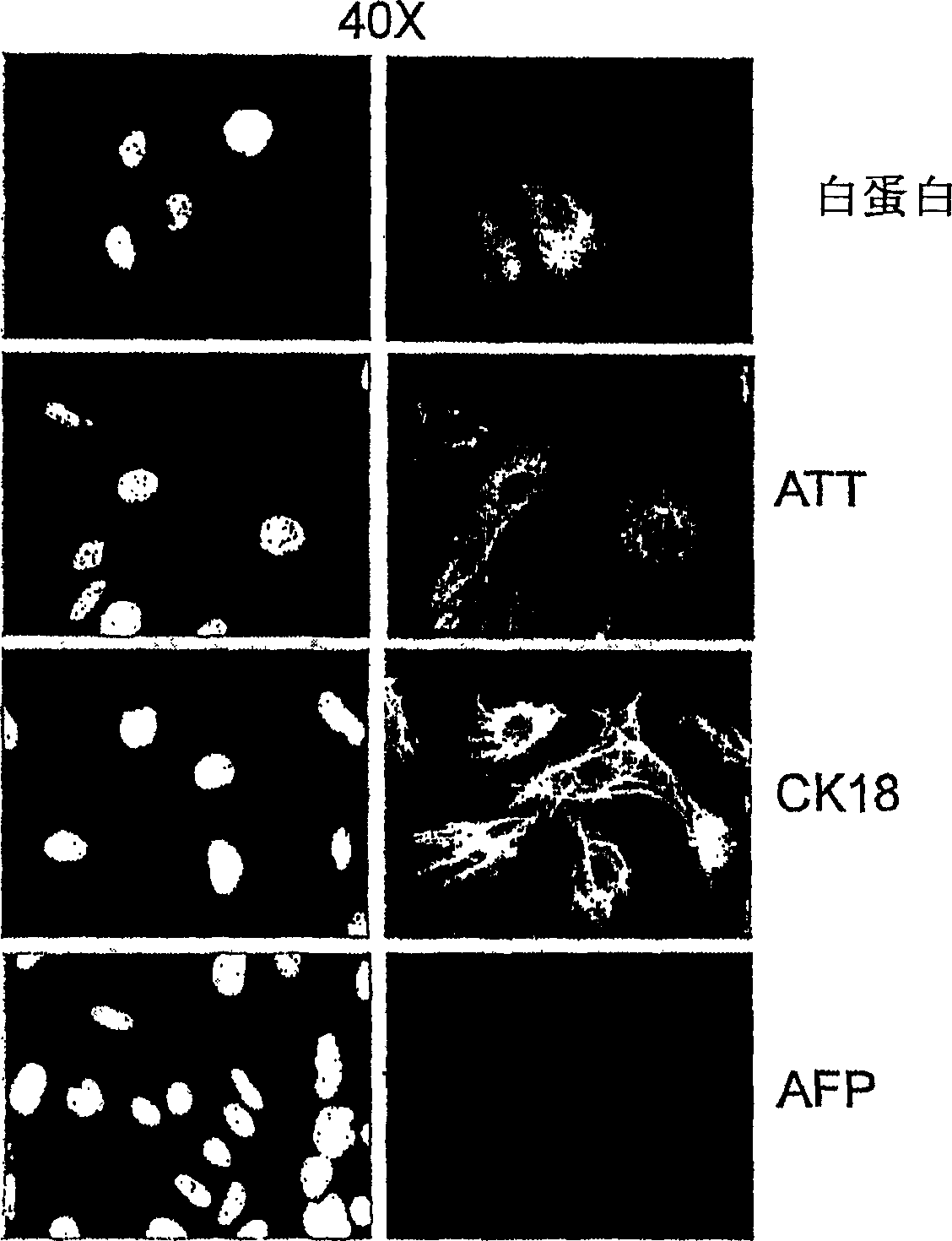
[0202]On day 6 after plating in differentiating agents, cells were analyzed for marker expression by RT-PCR and immunocytochemistry according to the methods described above. Glycogen content in these ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com