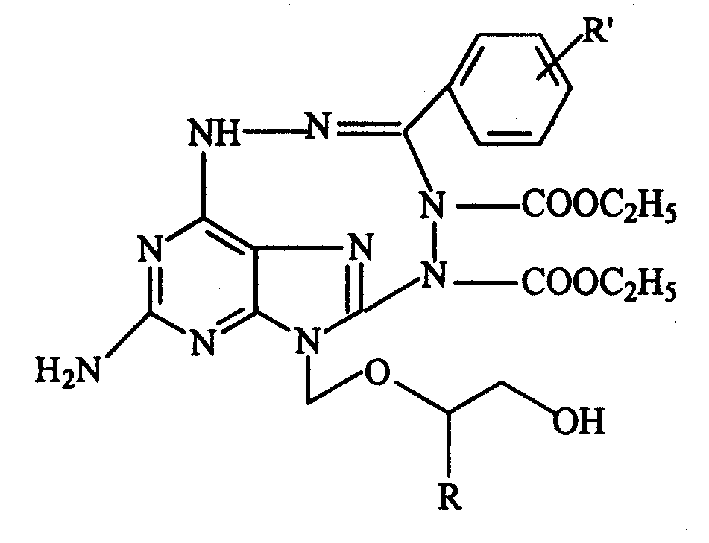
New condensed tricyclonucleoside compound containing acyclovir fragment and its preparation method
A nucleoside compound, compound technology, applied in the direction of organic chemistry, antiviral agent, etc., can solve the problems of low bioavailability, poor water solubility, etc., and achieve the effect of high biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047]9-(2-hydroxyethoxy)methyl-2-amino-6-N'-substituted benzylidene hydrazino-6-deoxy-9H-purine synthetic general method, with 9-(2-hydroxyethyl Oxy)methyl-2-amino-6-N'-benzylidenehydrazino-6-deoxy-9H-purine as an example: Reaction step 1
[0048] 9-(2-Hydroxyethoxy)methyl-2-amino-6-hydrazino-6-deoxy-9H-purine
[0049] Take vacuum-dried 9-(2-hydroxyethoxy)methyl-2-amino-6-chloro-6-deoxy-9H-purine (3.0g, 0.0105mol) and add it to 10ml of absolute ethanol, stir After dissolving, hydrazine hydrate (1.0 g, 0.020 mol) was added, and 2.45 g of white solid was precipitated after reacting for 1 hour, with a yield of 98%. m.p.: 179-180°C. EI-MS m / z 239 (M + ) 1 H-NMR (400MHz, DMSO-d 6 )δ3.47(t, 4H, OCH 2 CH 2 O), 4.41 (S, 2H, NHNH 2 , disappear after adding D2O), 4.63 (s, H, OH, disappear after adding D2O), 5.37 (s, 2H, NCH 2 O), 5.94(s, 2H, CNH 2 , disappeared after adding D2O), 7.81 (s, H, 8-H) elemental analysis (C 8 h 13 N 2 o 2 ):
[0050] C% H% N% Calculate...
Embodiment 2
[0054] The synthetic general method of the tricyclic nucleoside compound containing nine-membered heterocycle, with 3-amino-14-(2-hydroxyl-ethoxymethyl)-8-(4-methoxyl-phenyl) 2,4 , 6, 7, 9, 10, 12, 14-octaaza-tricyclo [9.2.2.0 5,13 ]Tetradecane-1,3,5(13),7,11-pentaene-9,10-dicarboxylate diethyl ester as an example:
[0055] Take vacuum-dried 9-(2-hydroxyethoxy)methyl-2-amino-6-N'-(4-methoxybenzylidene)-hydrazino-6-deoxy-9H-purine (0.15 g, 0.42mmol), put into a 25ml eggplant-shaped flask, add 8ml of diethyl azodicarboxylate (DEAD) and 2ml of methanol, heat to 120°C, and react for 1 hour, the solution is clear, and thin-layer chromatography checks (silica gel prefabricated plate, developer dichloromethane:methanol=10:1) shows that reaction is complete, and the R of product f = 0.78. Silica gel column chromatography with ethyl acetate as the eluent gave 0.132 g of a light yellow solid with a yield of 61%. m.p.: 214-215°C. EI-MS m / z 529 (M + ) 1 H-NMR (400MHz, DMSO-d 6 )δ1...
Embodiment 3
[0058] 3-Amino-14-(2-hydroxy-ethoxymethyl)-8-(phenyl)-2,4,6,7,9,10,12,14-octaaza-tricyclo[9.2.2.0 5.13 ] Tetradecane-1,3,5(13), 7,11-pentaene-9,10-dicarboxylate diethyl ester (C-1)
[0059] Take vacuum-dried 9-(2-hydroxyethoxy)methyl-2-amino-6-N'-benzylidene-hydrazine-6-deoxy-9H-purine (0.15g, 0.495mmol), put Put into a 25ml eggplant-shaped flask, add DEAD8ml and anhydrous methanol 2ml, heat to 120°C, react for 1 hour and then the solution is clear, TLC inspection (silica gel prefabricated plate, developer dichloromethane:methanol=10:1) Shows complete disappearance of reactants. Column chromatography, the eluent was ethyl acetate, and 0.105 g of light yellow solid was obtained with a yield of 46%. m.p.: 122-123°C. EI-MS m / z 499 (M + ) 1 H-NMR (400Hz, DMSO-d 6 )δ1.185(t, 3H, OCH 2 CH 3 ), 1.22(t, 3H, OCH 2 CH 3 ), 3.49(t, 2H, OCH 2 CH 2 O), 3.57(t, 2H, OCH 2 CH 2 O), 4.15(q, 2H, OCH 2 CH 3 ), 4.20 (q, 2H, OCH 2 CH 3 ), 4.585 (s, H, OH, adding D 2 Disappear af...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com