HLA complement-dependent cytotoxcity antibody detection method using ELIA as basis and its kit
A kit and antibody technology, applied in the field of CDC effect evaluation, can solve problems such as insufficient satisfaction, and achieve the effect of simple and easy to master method, low cost and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
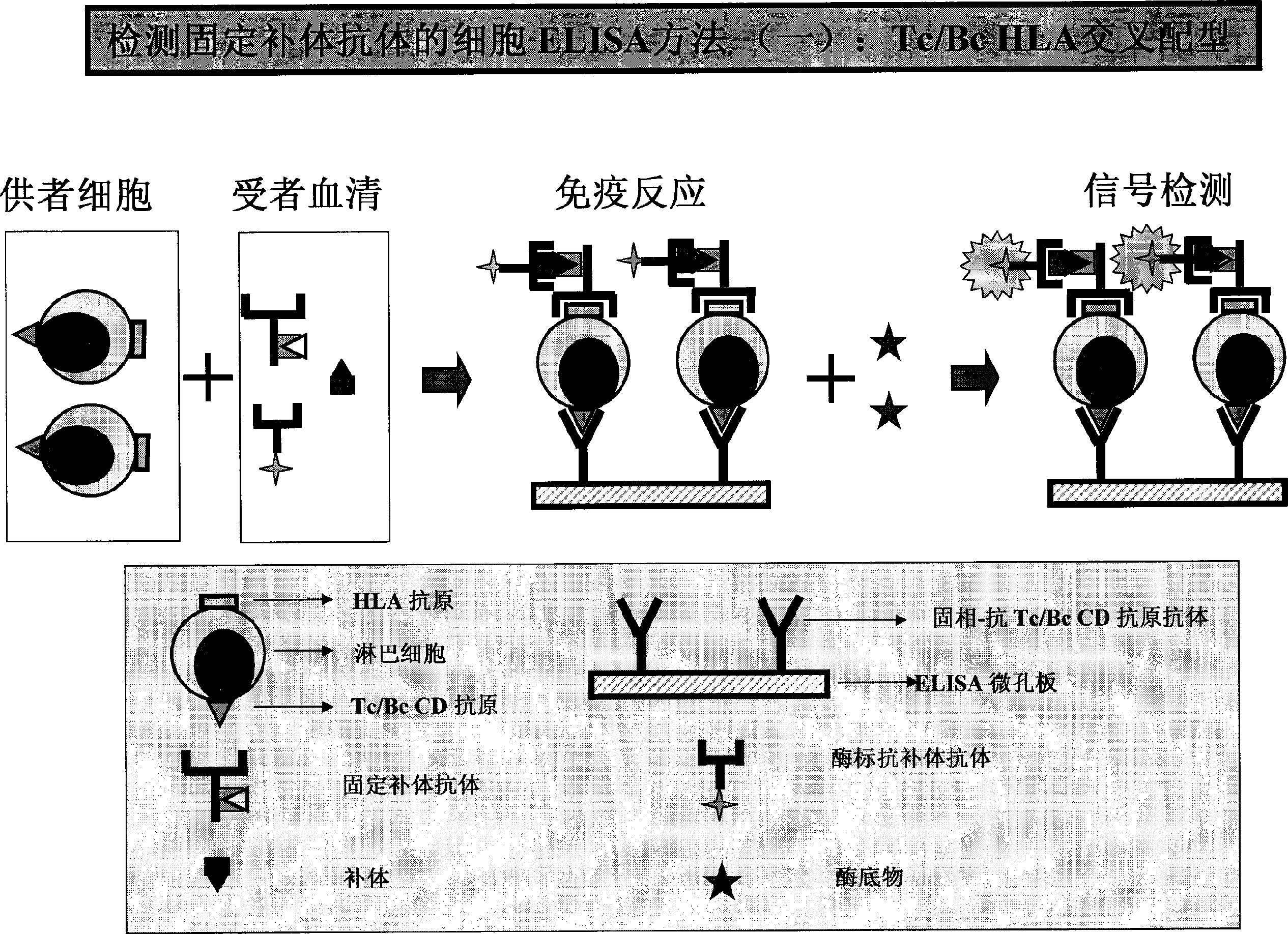
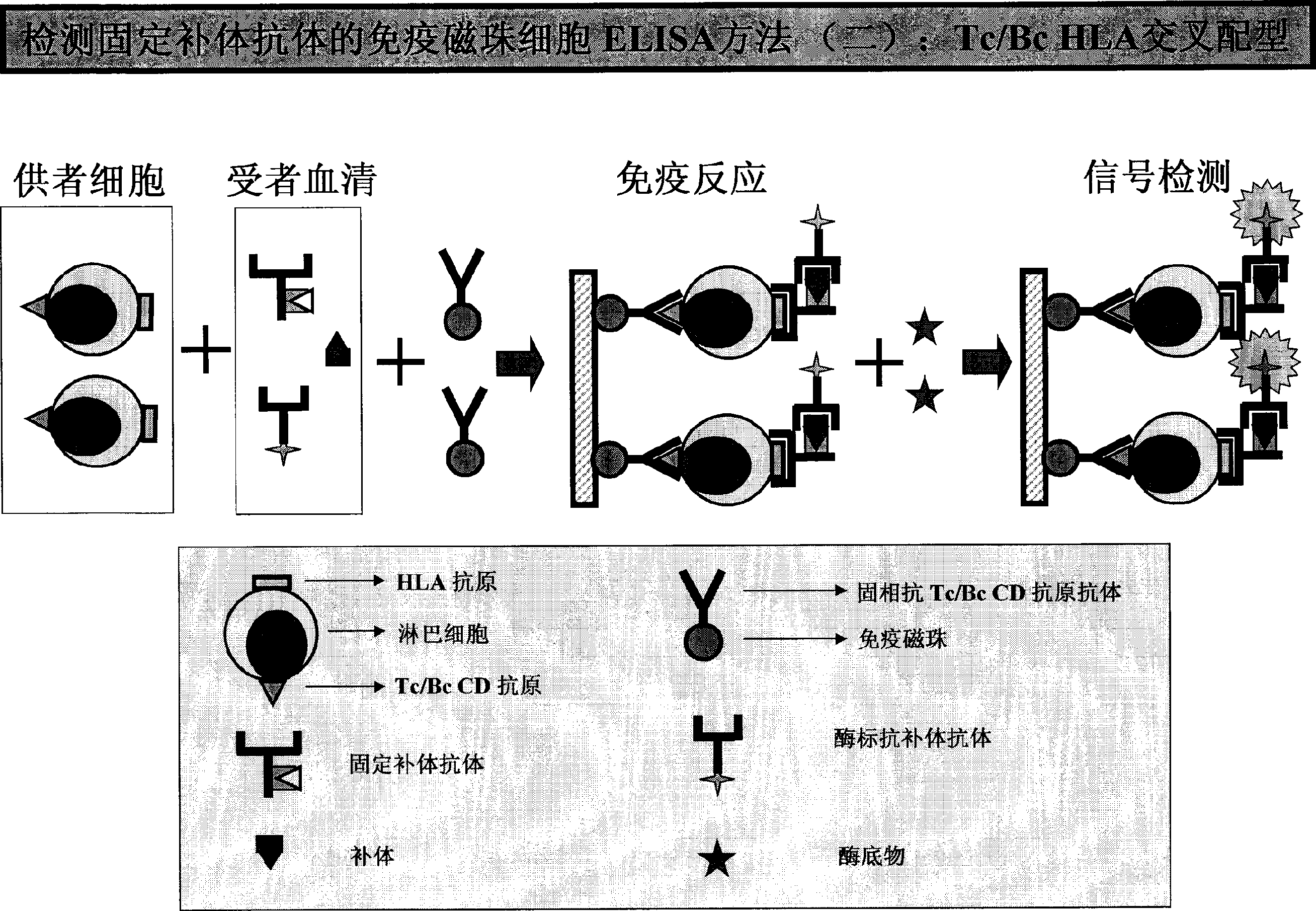
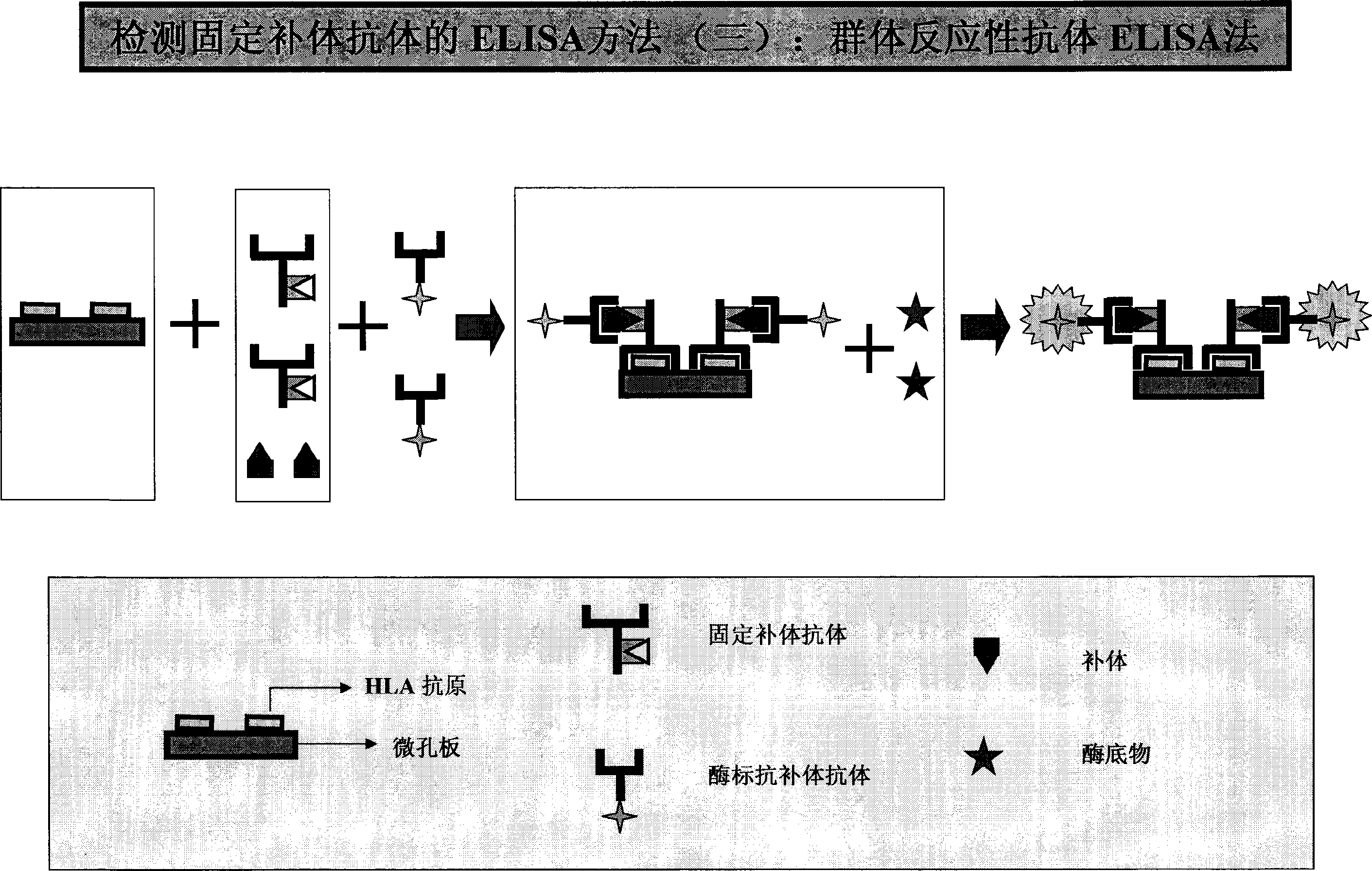
example 4 HLA-I or / and HLA-IIELISA approach ( 1 ( or )HLA-I or / and HLA-II。( 2 example 3 ( 3 、( 4 ( 3 1 ; or 1 。;, and 2-3, 1 。( 4 example 3 ( 8 、( 9 、( 10 。 example 5 HLA CFAbs approach (CFAbs-PRA)( 1 PRA preparation :HLA, 1 (0.01-3×106/);CELISA; or HLA or 。( 2 PRA 1 1 , and or ; example 3 ( 4 。( 3 or HLA;;;。( 4 HRP-C1qAb or HRP-C3Ab(), 1 ()。( 5 1 HRP,OD。( 6 :ODCFAbs;PRA 100 ;:CFAbs÷PRA×100=(PRA%) example :CFAbs25;PRA50;PRA%50%。 example 6 HLA-I and HLA-IICFAbs( 1 HLA-I or / and HLA-II or , example ,HLA-I or / and IICFAbs。( 2 HLA-ICFAbsHLA-I。( 3 HLA-I or II>95%HLA。 example 7
[0032] Or the total lgG on the surface of B lymphocytes can be measured to achieve simultaneous determination of CFABS and Non-CFAbs. Example 3: ELISA method for HLA Class I / IICFAbs (1) Purified HLA Class I or / and Class II antigens are coated with a microtiter plate at 37°C for 1-4 hours or 4°C overnight. Aspirate and discard the package. By liquid. (2) Block the microplate with a buffer (blocking solution) containing a certain amount of non-specific protein (such as BSA, skimmed milk powder, calf serum, etc.), with the same time and temperature as step (1), and then aspirate the remaining blocking liquid. (3) Add 50 μl each of negative control serum, positive control serum, background buffer and test serum into the corresponding microplates (also can be serum diluted with buffer). (4) Incubate the above-mentioned microplate at 37°C or 4°C for 30 minutes to 3 hours or 4°C overnight. (5) Aspirate and discard the reaction solution, and wash each well of the microplate with a deterge...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com