Ethene polymerization catalyst for homemade comonomer, preparation process and application thereof
A technology for ethylene copolymerization and comonomer, which is used in the field of ethylene copolymerization and the preparation of supported catalysts, and can solve the problems of limited copolymerization performance of comonomers and catalysts, short branch chains of comonomers, and expensive comonomers.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
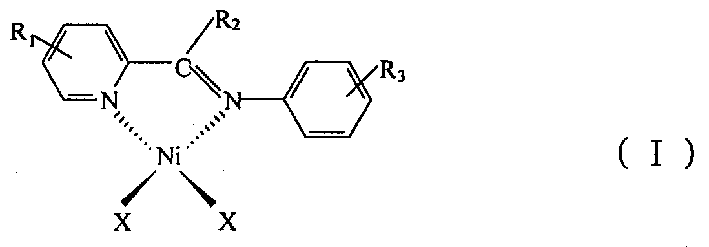
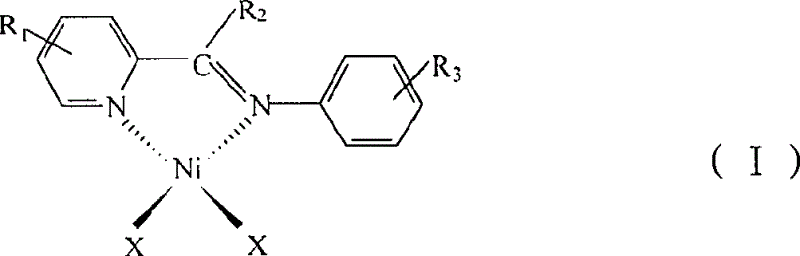
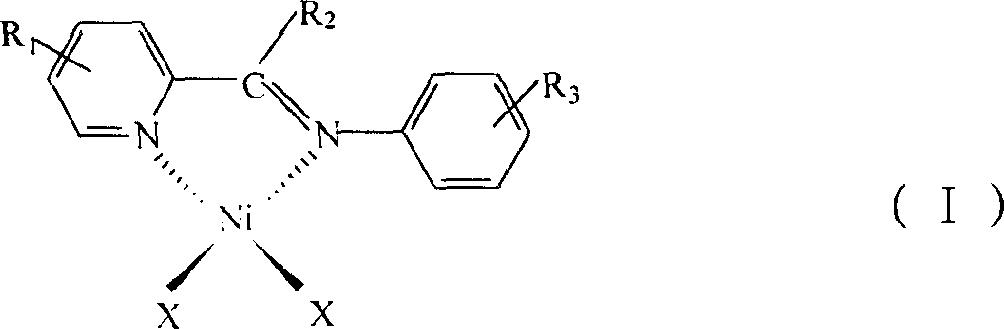
[0038] Preparation of oligomerization catalyst 2-(2,6-diisopropylphenylimine)picoline nickel dibromide [PyCH=NPh i PR 2 ]NiBr 2
[0039] (1) Preparation of 2-(2,6-diisopropylphenylimine)picoline PyCH=NPh i PR 2
[0040] In 20ml methanol, add 0.01mol of 2,6-diisopropylaniline (Sweden, produced by ACR S company) and make it dissolve, then add 0.01mol of 2-pyridinecarbaldehyde (Sweden, produced by ACR S company), and 5 drops of formic acid (China, Beijing Chemical Plant). Heat to reflux at 60°C for 3 hours. Cool to 30° C., remove the solvent under reduced pressure, wash twice with 10 ml of ethanol, and dry to obtain 1.81 g of 2-(2,6-diisopropylphenylimine)picoline, with a yield of 68% by weight.
[0041] (2) Preparation of catalyst [PyCH=NPh i PR 2 ]NiBr 2
[0042] Add 1.5 g of nickel dibromide (produced by ACR S in Sweden) to 25 ml of ethylene glycol dimethyl ether (DME, produced by ACR S in Sweden), and reflux at 85° C. for 4 hours while stirring. Cool to room tempe...
example 2
[0045] Preparation of 2-(2,6-diisopropylphenylimine)methyl-6-picoline nickel dibromide [MePyCH=NPh i PR 2 ]NiBr 2
[0046] (1) Preparation of 2-(2,6-diisopropylphenylimine)methyl-6-picoline MePyCH=NPh i PR 2
[0047]In 20ml of methanol, add 0.01mol of 2,6-diisopropylaniline to dissolve, then add 0.01mol of 6-picoline-2-carbaldehyde (produced by ACRS in Sweden) and 5 drops of formic acid. Heat to reflux at 65°C for 16 hours. Cool to 30°C, remove the solvent under reduced pressure, wash twice with 10ml ethanol, and dry to obtain 1.85 grams of 2-(2,6-diisopropylphenylimine)methyl-6-methylpyridine, with a yield of 66% by weight.
[0048] (2) Preparation of [6-MePyCH=NPh i PR 2 ]NiBr 2
[0049] In 10ml of dichloromethane, add 1mmol (DME) NiBr 2 , stirred to dissolve it, and then added 10 ml of a dichloromethane solution of 1.05 mmol 2-(2,6-diisopropylbenimine) methyl-6-picoline. Heated to 80°C and refluxed for 4 hours, filtered after cooling, washed the solid twice wi...
example 3
[0051] Preparation of 2-(2,4,6-trimethylbenzimine)picoline nickel dibromide [PyCH=NPhMe 3 ]NiBr 2
[0052] (1) Preparation of 2-(2,4,6-trimethylimine)picoline PyCH=NPhMe 3
[0053] Add 0.01 mol of 2,4,6-trimethylaniline (produced by ACRS in Sweden) to 20 ml of methanol, stir and dissolve, then add 0.01 mol of 2-pyridinecarbaldehyde and 5 drops of formic acid. Heat to reflux at 40°C for 36 hours. Cool to 25°C, remove the solvent under reduced pressure, wash twice with 10ml ethanol, and dry to obtain 1.3 g of 2-(2,4,6-trimethylbenimine)picoline PyCH=NPhMe 3 , the yield was 60% by weight.
[0054] (2) Preparation of [PyCH=NPhMe 3 ]NiBr 2
[0055] Add 1mmol NiBr in 10ml acetonitrile 2 , stirred, and then added 10 ml of 1.05 mmol 2-(2,4,6-trimethylbenimine)picoline in acetonitrile. Heat to 80°C and reflux for 36 hours, filter after cooling, wash the solid once with 5ml of ether and hexane, and dry in vacuo to obtain 0.4g of catalyst C[PyCH=NPhMe 3 ]NiBr 2 , the yield w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melt flow index | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com