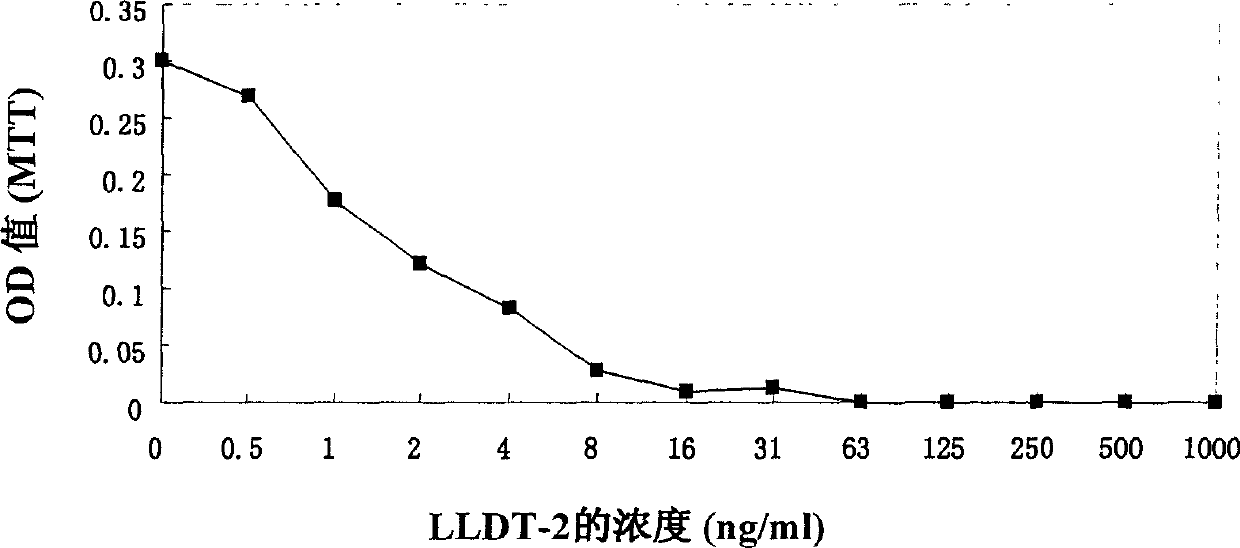
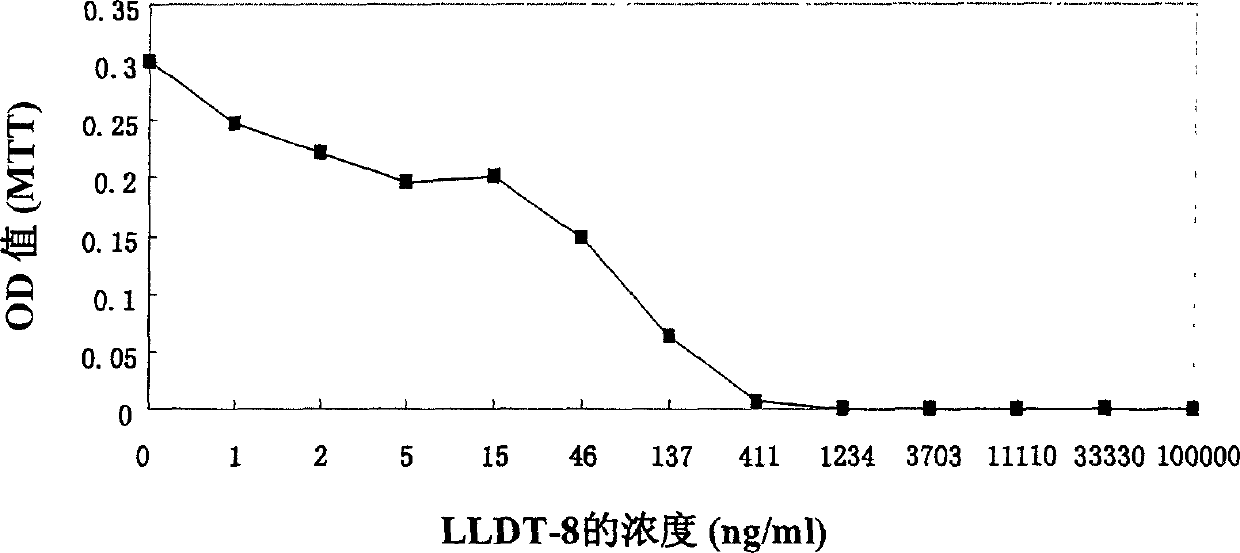
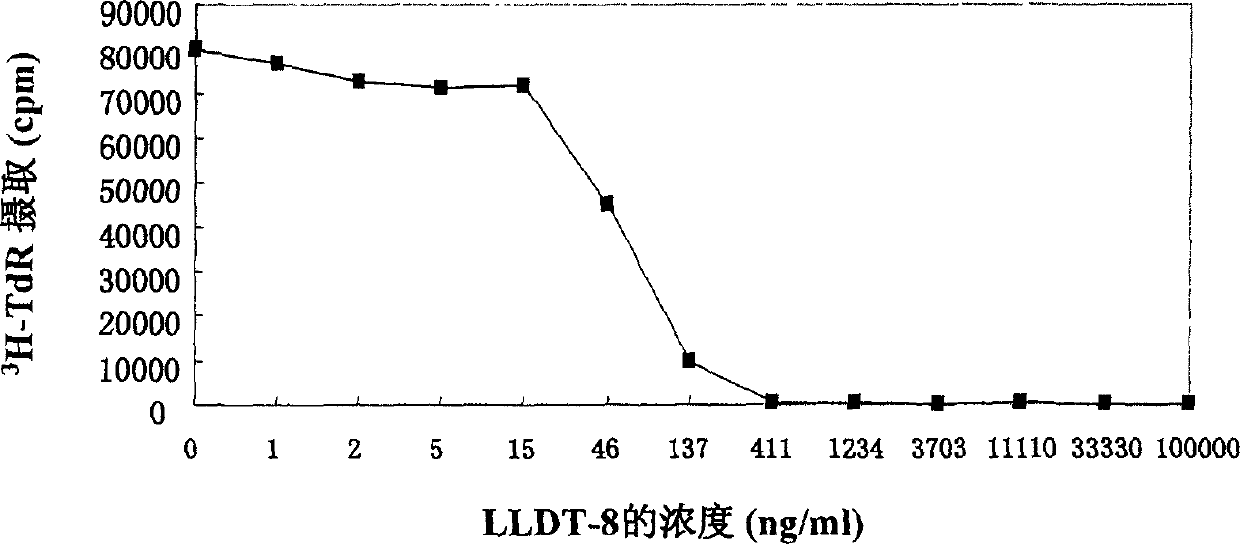
Triptolide alcohol derivative and its use
A technology for triptolide and derivatives is applied in the field of structural modification and activity research of active ingredients of natural medicines, and can solve problems such as limited application, toxic and side effects of triptolide, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1 (5R)-5-hydroxy triptolide ketone
[0065]
[0066] Dissolve triptolide (374mg, 1.04mmol) in 20ml DMSO, then add SeO 2 (461mg, 4.16mmol), heated under slight reflux for 10 hours. The reaction system was cooled to room temperature, filtered, the filter residue was washed with ethyl acetate, the washing liquid was combined with the filtrate, and the solvent was evaporated under reduced pressure. The residue was saturated with Na 2 CO 3 Dissolved, extracted with ethyl acetate, the organic phase was washed with water and saturated brine, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain a white solid (5R)-5-hydroxytriptolide ketone (319mg, 0.85mmol, yield 82%).
[0067] 1 H-NMR (DMSO-d 6 , 400MHz) δ4.88(m, 2H), 4.11(d, J=2.9Hz, 1H), 4.08(d, J=2.9Hz, 1H), 3.42(d, J=4.4Hz, 1H), 2.24( Septet, J=6.9zHz, 1H), 1.96-2.20(m, 4H), 1.83(ddd, J=6.4, 11.7, 11.8Hz, 1H), 1.08(broad, dd, J=5.2, 12.4Hz, 1H ), 0.91(s,...
Embodiment 2
[0068] Example 2 (5R)-5-hydroxy triptolide and (5R)-5-hydroxy epitriptolide
[0069]
[0070] To a suspension of (5R)-5-hydroxytriptolide (20 mg, 0.053 mmol) dissolved in 5 mL of methanol was added NaBH 4 (8mg, 0.21mmol), stirred for 2 hours, the reaction system became a colorless clear solution, the solvent was evaporated under reduced pressure, the residue was dissolved in ethyl acetate, washed with water and saturated brine respectively, dried over anhydrous sodium sulfate, and reduced pressure The solvent was evaporated to dryness, and the residue was separated by column chromatography (eluent: cyclohexane: ethyl acetate = 1:1) to obtain (5R)-5-hydroxytriptolide (7.5mg, 0.02mmol, yield 38 %) and (5R)-5-hydroxyepitriptolide (11.3 mg, 0.03 mmol, yield 56%).
[0071] (5R)-5-Hydroxytriptolide:
[0072] 1 H-NMR (DMSO-d 6 , 400Hz) δ5.32(s, 1H), 4.87(m, 2H), 4.57(br.s, 1H), 3.73(d, J=2.9Hz, 1H), 3.53(d, J=2.9Hz, 1H ), 3.38(s, 1H), 3.34(d, J=5.0Hz, 1H), 2.07-2.19(m, 4H), 1...
Embodiment 3
[0075] Example 3 Δ 5,6 - Dehydrotriptolide
[0076]
[0077] Add (5R)-5-hydroxytriptolide ketone (224mg, 0.60mmol) into a solution of anhydrous pyridine (4ml, 50.57mmol) in dichloromethane (10ml), stir to dissolve, and then add trifluoro Acetic anhydride (600 mg, 2.85 mmol) was reacted at room temperature for 12 hours, and after thin layer chromatography (cyclohexane: ethyl acetate = 1: 1) checked that the raw materials disappeared, the reaction was terminated, the solvent was distilled off under reduced pressure, and the residue was distilled off with water (20 ml ) was diluted, added ethyl acetate (40ml × 3) for extraction, the organic phase was washed with dilute sulfuric acid, saturated sodium bicarbonate solution and saturated brine respectively, dried over anhydrous sodium sulfate, and the solvent was evaporated to obtain a brownish-yellow oil. Analysis and separation (eluent: cyclohexane: ethyl acetate = 3: 1), recrystallization from cyclohexane-ethyl acetate to giv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com