Growth hormone and serum albumni recombinant fusion protein
A serum albumin, growth hormone technology, applied in the direction of serum albumin, growth hormone, albumin peptide, etc., can solve the problem of inactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
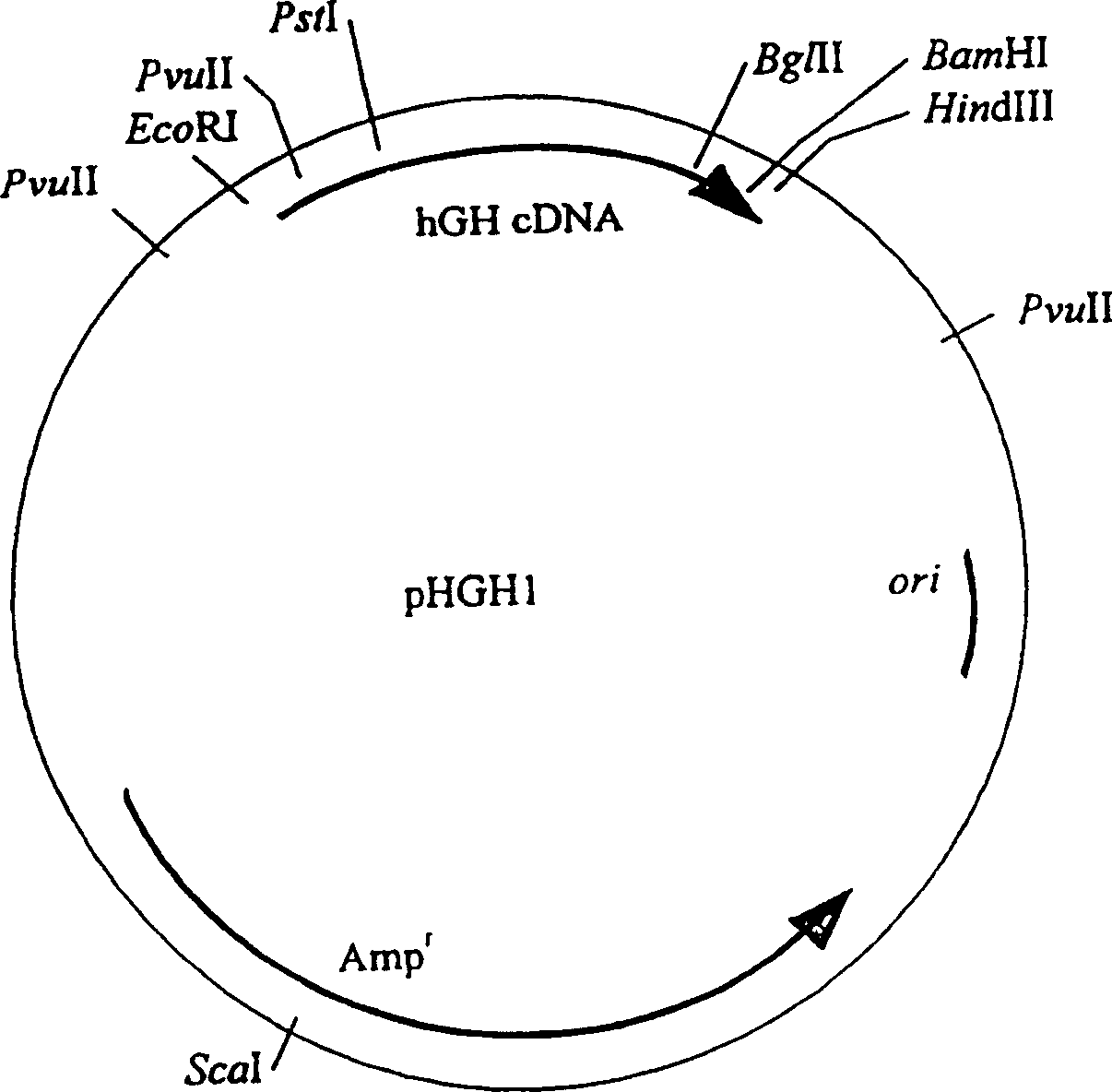
[0087] Example 1: Cloning of hGH cDNA.
[0088] The cDNA of hGH was obtained from the human pituitary gland cDNA library by the method of PCR amplification (catalogue number is HL1097V, (Clontech Laboratories, Inc). Two oligonucleotide primers suitable for hGH cDNA amplification: HGH1 and HGH2 use Biological system 380B (Applied Biosystems380B) oligonucleotide synthesizer synthesis.
[0089] HGH1: 5′-CCCAAGAATTCCCTTATCCAGGC-3′
[0090] HGH2: 5′-GGGAAGCTTAGAAGCCACAGGATCCCTCCACAG-3′
[0091] HGH1 and HGH2 differ from the corresponding part of the hGH cDNA sequence by two and three nucleotides, respectively (Fig. 1, Martial et al., 1979), thus, after PCR amplification, an EcoRI site was introduced at the 5'-end of the cDNA , a BamHI site was introduced at the 3′-end. In addition, there is a Hind III site in HGH2 immediately downstream of the hGH sequence.
[0092] Carrying out PCR amplification with Perkin-ELmer-Cetus Thermal Cycler 9600 amplification instrument and Perkin-El...
Embodiment 2
[0094] Example 2: Expression of hGH cDNA
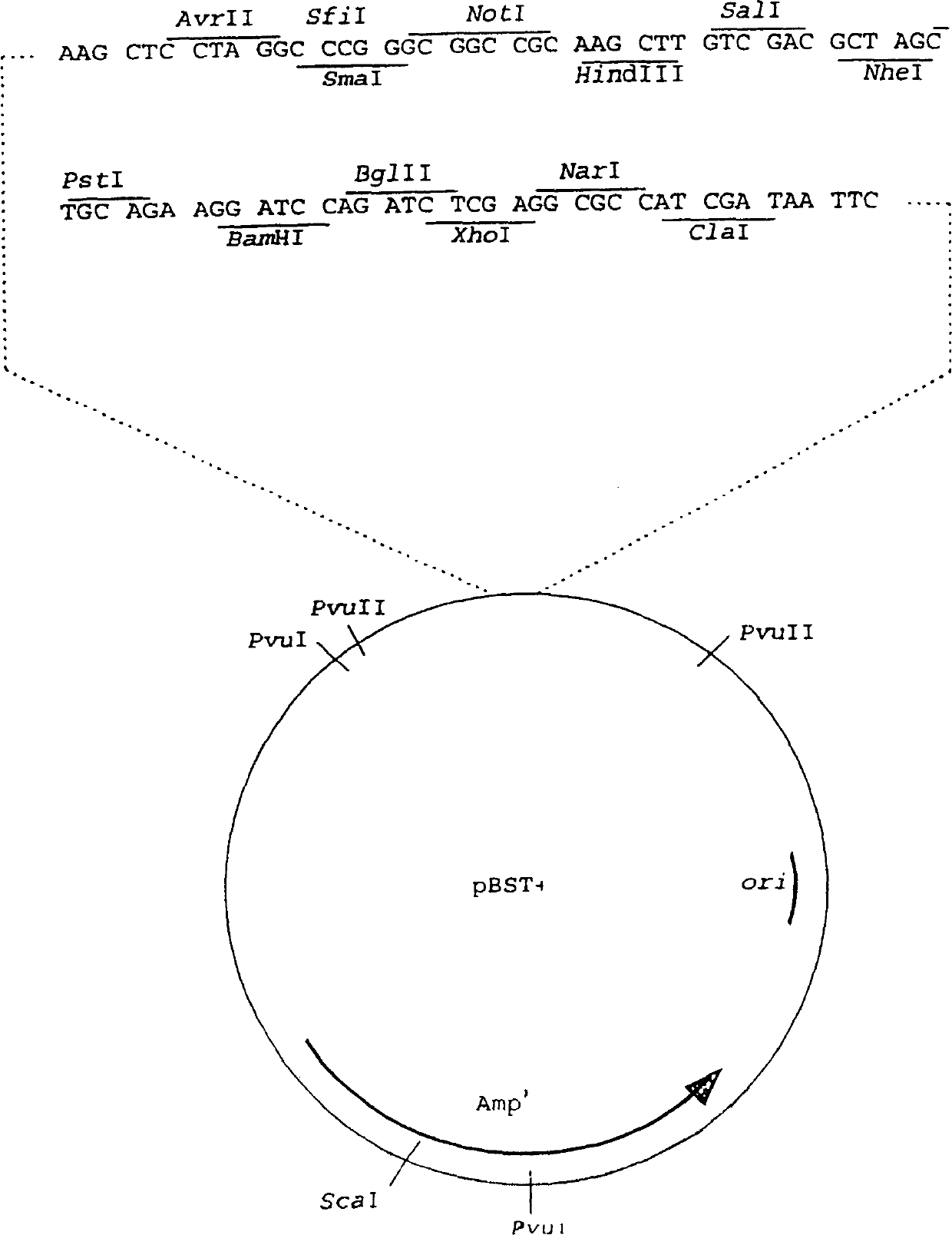
[0095] The polylinker sequence of the phagemid pBluescribe(+) (Stratagene) was replaced by an oligonucleotide linker formed by annealing two 75-mer oligonucleotides between the EcoRI and Hind III sites to form pBST ( +) (Figure 3). The new polylinker contains a unique NotI site (see Figure 3 for the full sequence of the polylinker region).
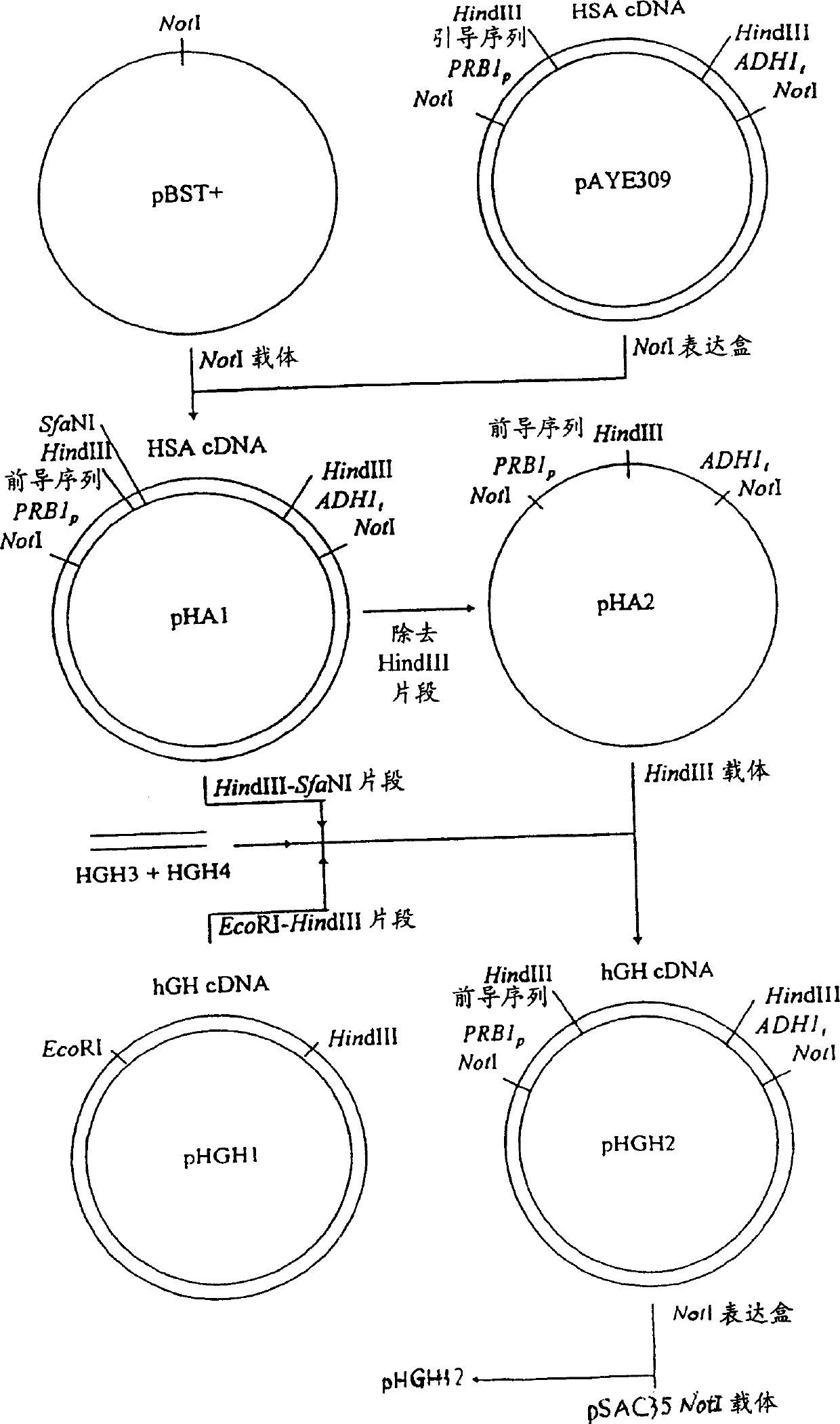
[0096] The NotI HSA expression cassette of pAYE 309 (EP 431 880) containing the PRB1 promoter, DNA encoding the HSA / MFα-1 hybrid leader sequence, DNA encoding HSA, and the ADH1 terminator was transformed into pBST(+) to form pHA1 (Figure 4). The coding sequence of HSA was removed from the plasmid by digestion with Hind III, and re-ligated to form pHA2 (Fig. 4).
[0097] Cloning of the hGH cDNA described in Example 1 provided the hGH coding region lacking the original hGH sequence and the first 8 base pairs (bp) of the mature hGH sequence. To construct an expression plasmid that secretes hGH in...
Embodiment 3
[0103] Embodiment 3: Cloning and expression of HSA-hGH fusion protein
[0104] To fuse HSA cDNA to the 5'-end of hGH cDNA, the pHA1 Hind III-Bsu 36I fragment (containing most of the HSA cDNA) and the EcoR1-Hind III fragment of pHGH1 were ligated by two oligonucleotides HGH7 and HGH8:
[0105] HGH7: 5′-TTAGGCTTATTCCCAAC-3′
[0106] HGH8: 5′-AATTGTTGGGAATAAGCC-3′
[0107] The Hind III fragment thus formed was cloned into pHA2 digested with Hind III to form pHGH10 (Fig. 5), and the expression cassette of this plasmid NotI was cloned into pSAC35 digested with Notl to form pHGH16 (Fig. 5).
[0108] pHGH16 was used for transformation into Saccharomyces cerevisiae DB1, and the culture supernatant was analyzed by the method in Example 2. A main band with a molecular weight of about 88KD was observed, corresponding to the combined size of HA and hGH. Western blotting with anti-HSA and anti-hGH antisera (Sigma) confirmed the presence of both components in the fusion protein.
[0109...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com