Medicine composition for treating cardiovascular and cerebrovascular disease and its prepararing method
A technology for cardiovascular and cerebrovascular diseases and compositions, which is applied in the field of pharmaceutical compositions for the treatment of cardiovascular and cerebrovascular diseases and its preparation, achieving the effects of clear active ingredients, definite curative effect, and avoiding unfavorable factors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
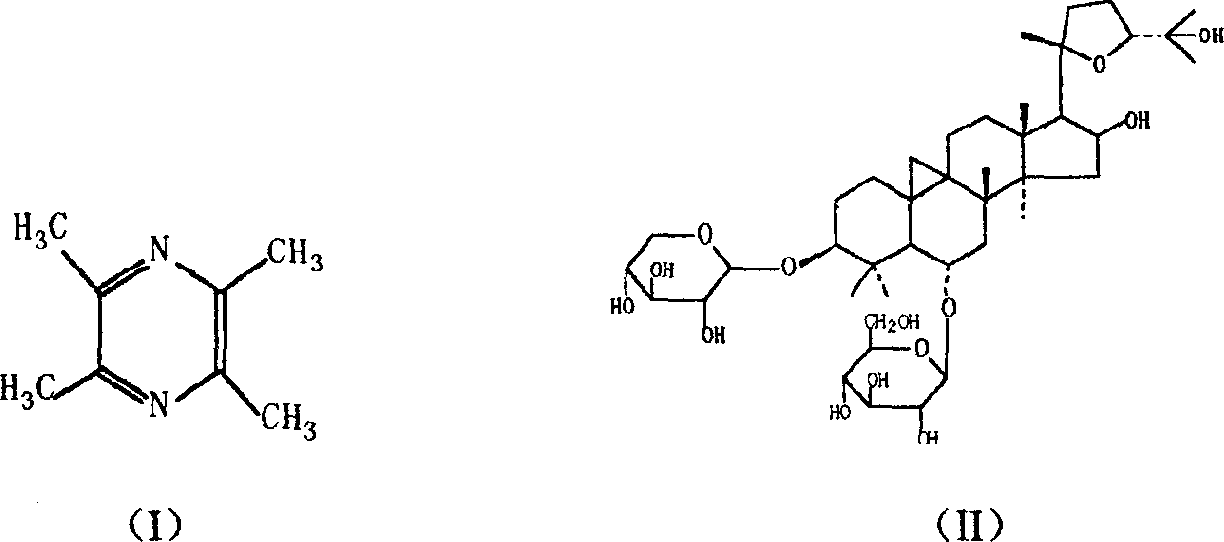
[0022] 50kg of traditional Chinese medicine Astragalus membranaceus, sliced, decocted 3 times with 10 times of water, 1 hour each time, filtered, combined decoction, passed through D201 macroporous resin column, washed with 200 liters of water and 100 liters of 30% ethanol to remove impurities, and then used 200 liters of ethanol with a volume content of 70% was eluted, and the ethanol eluate was collected, and the ethanol was recovered under reduced pressure. Add water to dissolve the residue, extract the aqueous solution with 20 liters of n-butanol in portions, combine the n-butanol extracts, wash twice with 10 liters of 2% sodium hydroxide aqueous solution, and wash twice with 5 liters of water, and recover the n-butanol Dried, pulverized, and degreased with 500ml of acetone in a Soxhlet extractor under reflux, and dried to obtain astragaloside. Detected according to the above method, the content of astragaloside IV is ≥ 20%.
[0023] Take 100g of ligustrazine phosphate and ...
Embodiment 2
[0025] 100kg of traditional Chinese medicine Astragalus membranaceus, sliced, add 15 times of water and decoct twice, 1.5 hours each time, filter, combine the decoction, after passing through the AB-8 macroporous resin column, wash with 400 liters of water and 200 liters of 40% ethanol to remove impurities , and then elute with 400 liters of 80% ethanol and collect the ethanol eluate. The eluate was dissolved in water after recovering ethanol under reduced pressure, and its aqueous solution was extracted with 40 liters of n-butanol in stages, and the n-butanol extracts were combined, washed with 30 liters of ammonia test solution (40ml concentrated ammonia water was added to make 100ml) in stages. After recovering n-butanol, dry and pulverize, and degrease with 2000 ml of acetone under stirring and reflux. After cooling, filter, wash with an appropriate amount of acetone, and dry to obtain astragaloside. The content of astragaloside IV was detected to meet the requirements. ...
Embodiment 3
[0028] 100kg of traditional Chinese medicine Astragalus membranaceus, sliced, add water 10 times and decoct 3 times, each time for 1 hour, filter, combine the decoction, pass through DM-301 macroporous resin column, wash with 400 liters of water and 200 liters of 30% ethanol to remove impurities Afterwards, elute with 400 liters of 75% ethanol and collect the ethanol eluate. Ethanol was recovered under reduced pressure and dissolved in water, and the aqueous solution was extracted with 40 liters of n-butanol in portions. After the n-butanol extracts were combined, they were washed with 20 liters of 5% aqueous sodium carbonate solution and then washed with 20 liters of water. Recover n-butanol from the extract and dry it, then use 1000ml of acetone to reflux in a Soxhlet extractor to degrease and dry to obtain astragaloside. The content of astragaloside IV was detected to meet the requirements.
[0029] Get 50g of ligustrazine phosphate and 150g of astragaloside, add 100g of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com