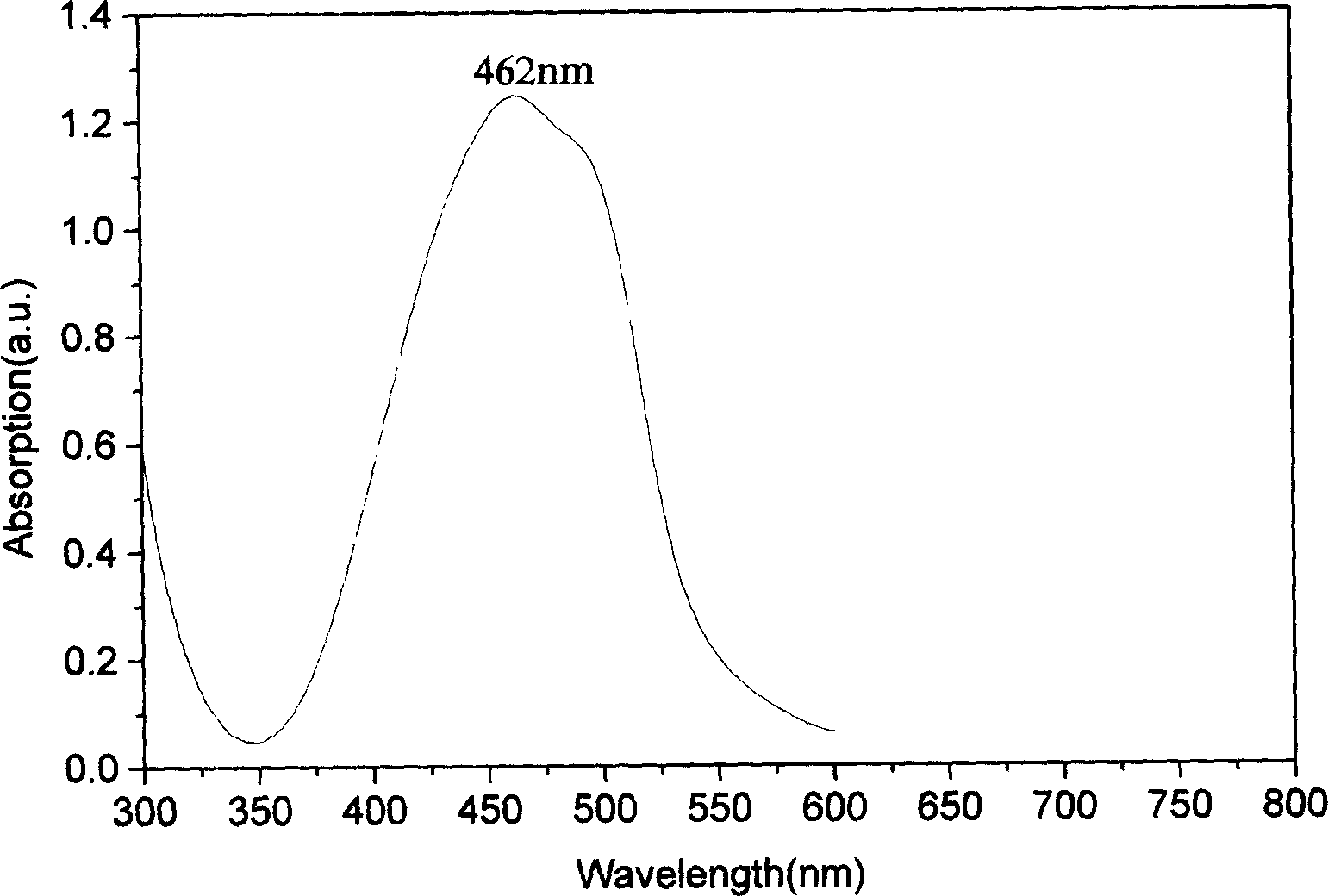
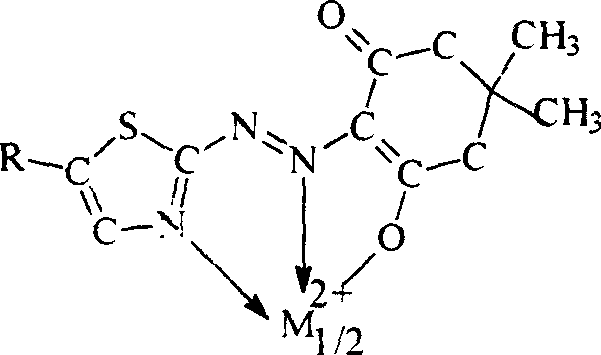
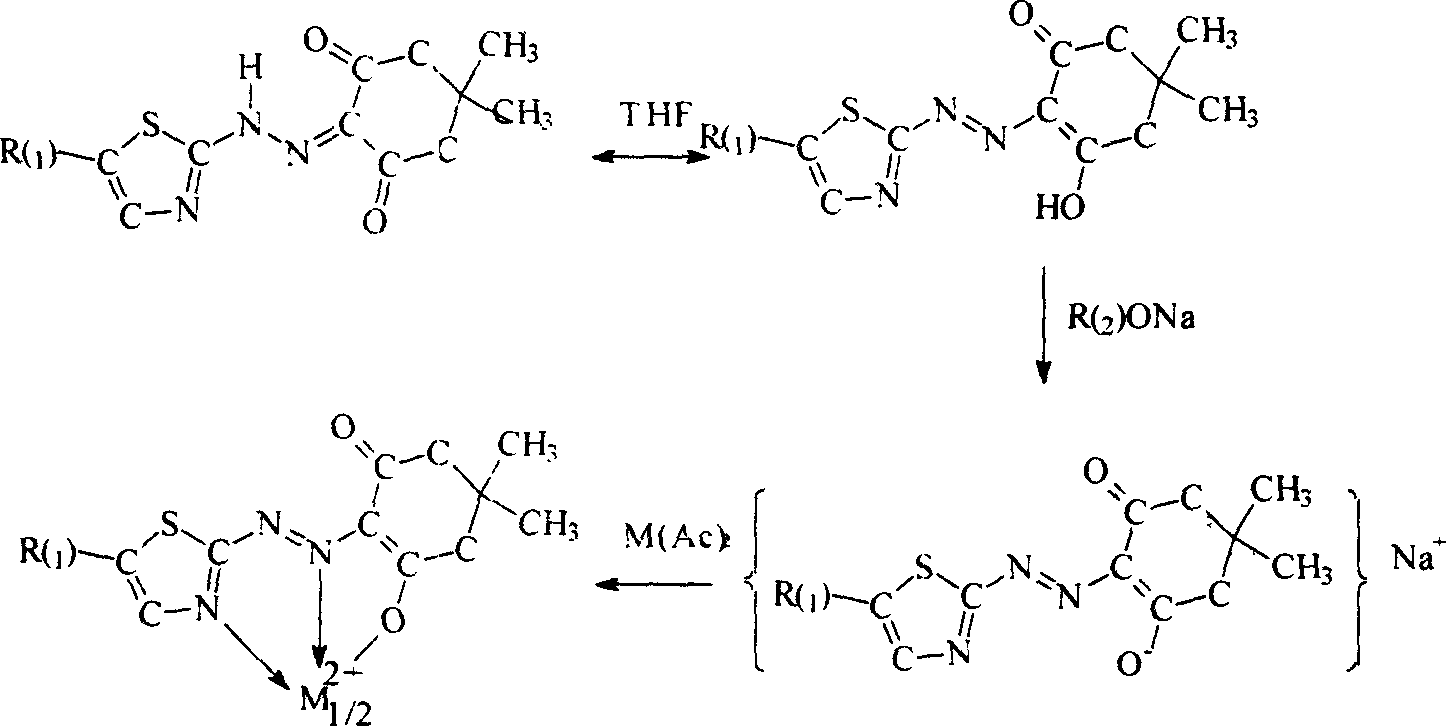
Metal chelate of short wavelength, and preparation method
A metal chelate, short-wavelength technology, used in the complex metal compounds of azo dyes, chemical instruments and methods, optical recording/reproducing, etc., can solve the problem of inability to use lasers, and achieve easy control of reaction conditions. , the effect of high yield and excellent photothermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the synthesis of α-(4-methyl-2-azothiazole ring)-β-(4', 4'-dimethylcyclohexanedione) nickel chelate
[0031] Its synthetic steps are as follows:
[0032] ① Weigh the substances according to the following weight percentages: α-(4-methyl-2-azothiazole ring)-β-(4',4'-dimethylcyclohexanedione) compound, tetrahydrofuran, sodium methoxide, The weight percentages of nickel acetate and methyl alcohol are respectively: 6%: 66%: 15%: 3%: 10%;
[0033] ②Add α-(4-methyl-2-azothiazole ring)-β-(4',4'-dimethylcyclohexanedione) compound into tetrahydrofuran, stir to dissolve it, and then add methanol Sodium solution and stirred at room temperature for 10 minutes, then slowly added nickel acetate and methanol solution, and reacted for about 1-3 hours;
[0034] ③ After the reaction is complete, add a certain amount of water, stir for a while and let it stand for 30 minutes, filter to obtain a large amount of precipitate, filter and dry.
[0035] Yield: 80%
Embodiment 2
[0036] Embodiment 2: the synthesis of α-(4-methyl-2-azothiazole ring)-β-(4', 4'-dimethylcyclohexanedione) copper chelate
[0037] The synthesis steps are as follows:
[0038] ① Weigh the substances according to the following weight percentages: α-(4-methyl-2-azothiazole ring)-β-(4',4'-dimethylcyclohexanedione) compound, tetrahydrofuran, sodium ethylate, The weight percentages of copper acetate and ethanol are respectively: 2%: 65%: 10%: 2%: 5%;
[0039] 2. Step is the same as Example 12. The difference is that nickel acetate is changed to copper acetate;
[0040] ③The steps are the same as Example 1③.
[0041] Yield: 85%.
Embodiment 3
[0042] Example 3: Synthesis of α-(4-tert-butyl-2-azothiazole ring)-β-(4', 4'-dimethylcyclohexanedione) copper chelate
[0043] Its synthetic steps are as follows:
[0044] ①Weigh the substance according to the following weight percentage: α-(4-tert-butyl-2-azothiazole ring)-β-(4',4'-dimethylcyclohexanedione) compound, tetrahydrofuran, sodium methoxide , copper acetate and methyl alcohol are respectively: 3%: 70%: 15%: 3%: 9%;
[0045] 2. Step is the same as Example 12. The difference is that nickel acetate is changed to copper acetate;
[0046] ③The steps are the same as Example 1③.
[0047] Yield: 86%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com