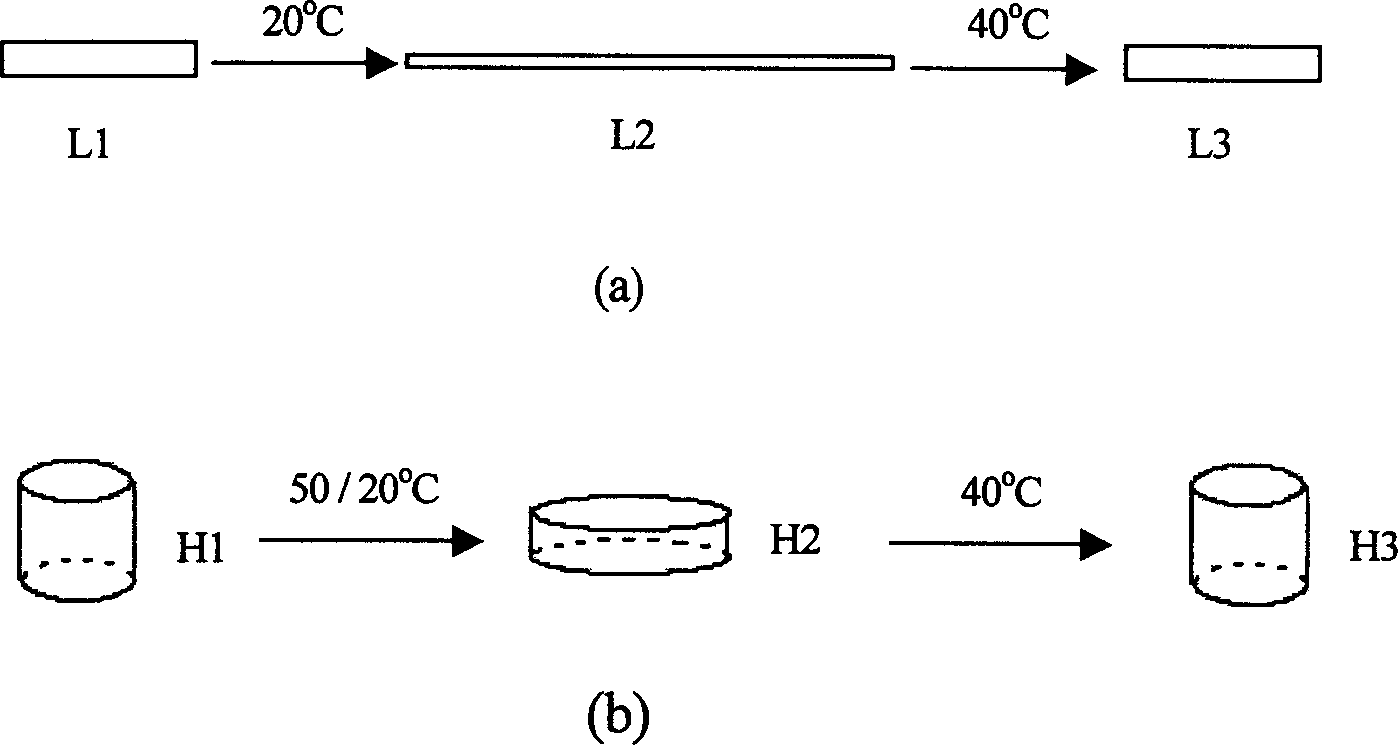
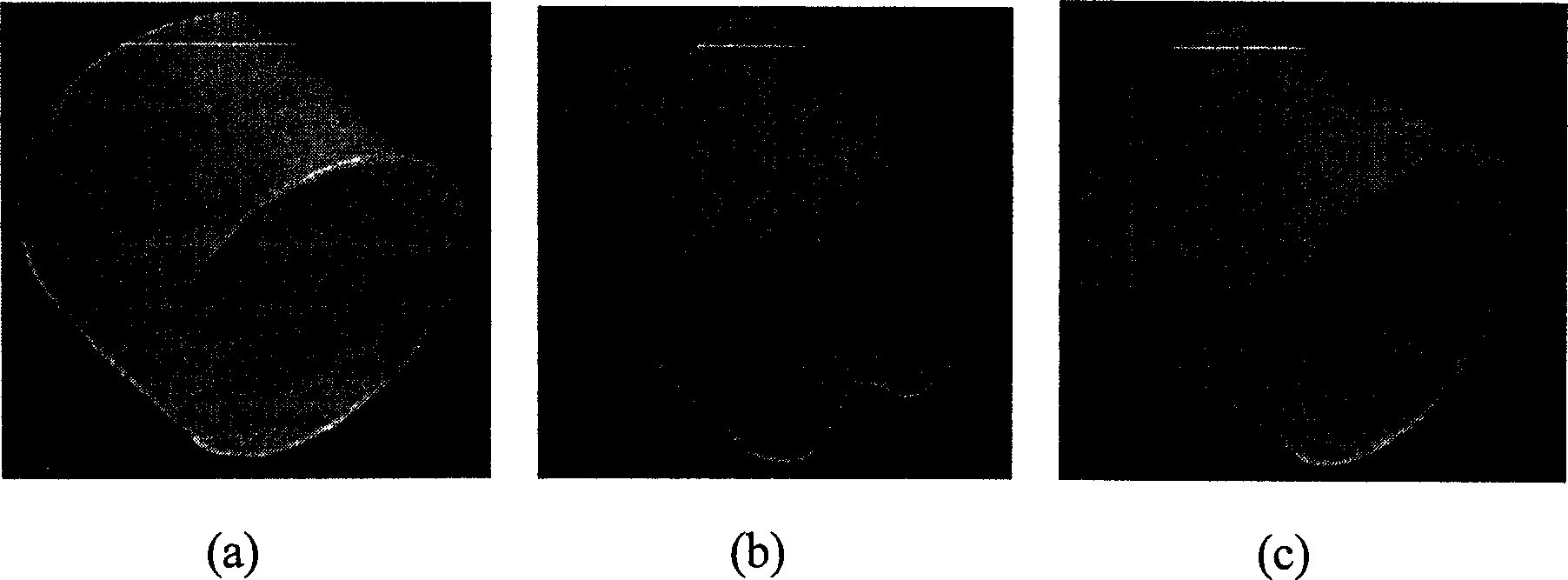
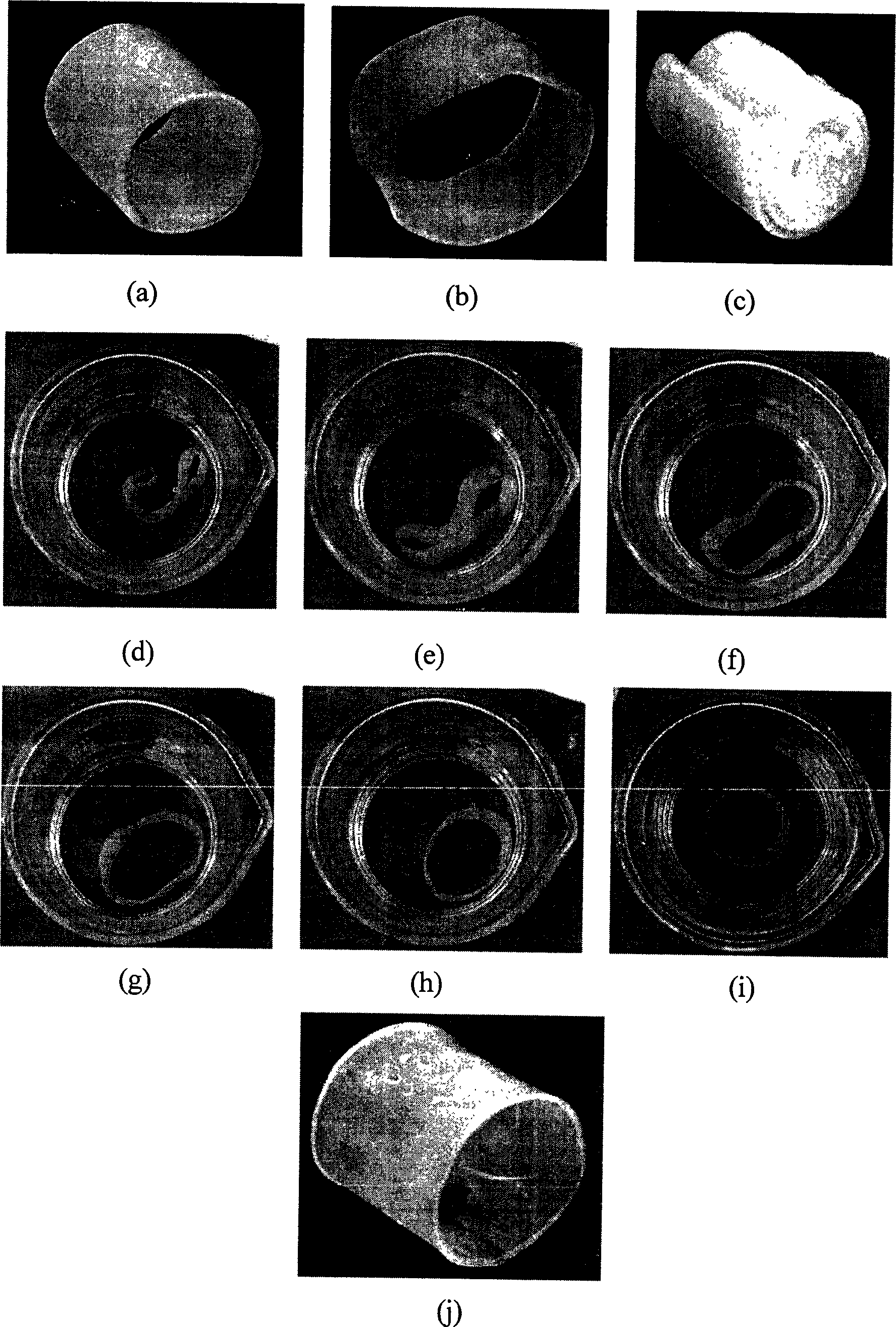
Shape memory material based on poly(e-caprolactone), preparation and metod of application
A memory material, caprolactone technology, applied in the field of medical polymer materials, can solve problems such as no shape memory function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]The concentration of 114g ε-caprolactone, 1.4g ethylene glycol and 5ml is 0.1molL -1 Sn(Oct) 2 Add the / toluene solution successively into a glass bottle that has been fully dried and equipped with a magnetic stirring bar, add an equal volume of toluene with a syringe, stir evenly, put it in a constant temperature oil bath at 120°C, heat the reaction for 24 hours, and pour it after cooling At room temperature, a toluene solution of poly(ε-caprolactone) (PCL) with double-terminated hydroxyl groups was obtained.
[0029] Add the calculated amount of 2,4-toluene diisocyanate (TDI) to the solution, heat and stir at 65°C for 15 minutes, then add the calculated amount of ethylene glycol (EG), so that the molar number of TDI is exactly equal to that of PCL and EO sum of moles. The reaction was continued to stir at 65°C for 3 hours, then the reaction product was dissolved in chloroform, precipitated with hexane, and dried under vacuum at 40°C for 24 hours.
[0030] By changin...
Embodiment 2
[0036] The reaction conditions in Example 1 are adopted, but in the first step of PCL synthesis, after obtaining the PCL toluene solution with double-terminal hydroxyl groups, instead of directly carrying out the next step reaction, PCL is precipitated with n-hexane, washed, and vacuum-dried. Obtain pure PCL prepolymer. Then dissolve the PCL prepolymer in toluene, add 0.1molL -1 Concentration of Sn(Oct) 2 / toluene for the next reaction. Compared with Example 1, the advantage of this reaction procedure is that the molecular weight and functionality of the PCL prepolymer can be accurately determined, so that the second step reaction can be accurately controlled to obtain a higher molecular weight. However, the melting point, crystallization temperature and minimum deformation recovery temperature of the product are basically unchanged.
Embodiment 3
[0038] The reaction procedure of Example 1 was followed, but substituting 2,4-toluene diisocyanate for 2,6-toluene diisocyanate. The melting point, crystallization temperature and minimum deformation recovery temperature of the obtained product are in the same temperature range as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com