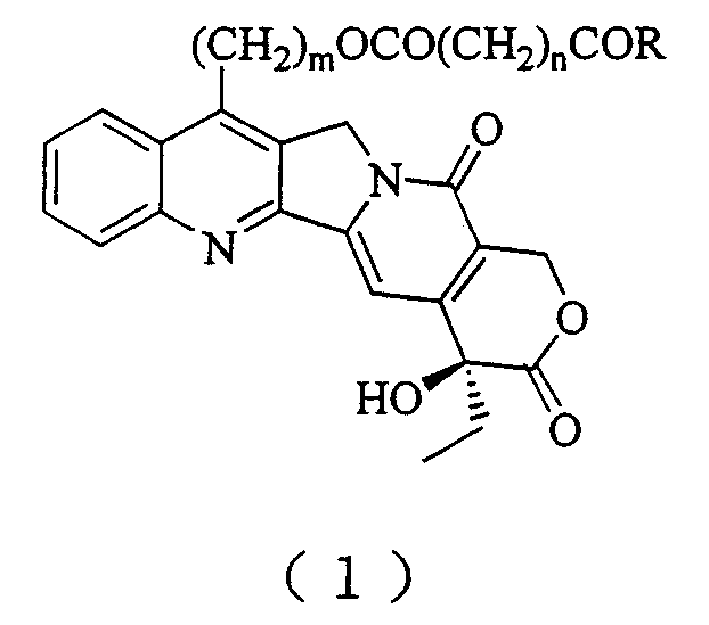
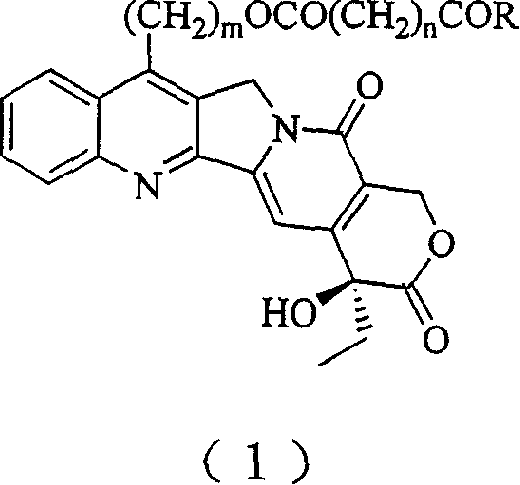
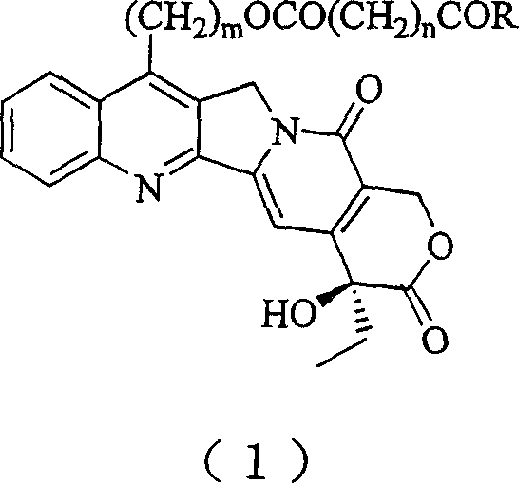
20(S) camptotheca alkaloid derivatives, their preparing methods and uses
A derivative, camptothecin technology, applied in the field of medicinal chemistry and therapeutics, can solve the problems of poor water solubility and high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of 7-Hydroxyethylcamptothecin
[0030] Dissolve 1g camptothecin in 30mL ethanol, stir, add 50mL 30% sulfuric acid solution dropwise under ice bath, then add 0.8g FeSO 4 ·7H 2 O, continue to slowly drop 9 mL of 30% H 2 o 2 , after the dropwise addition was completed, it was stirred overnight at room temperature. The reaction mixture was poured into distilled water, diluted to 800 mL, vacuum filtered, the filter cake was washed with distilled water, separated by silica gel column chromatography, and eluted with dichloromethane / methanol 50:1.2 to obtain 350 mg of a light yellow solid (produced rate 34%).
Embodiment 2
[0032] Preparation of 7-Hydroxyethylcamptothecin Succinate
[0033] Dissolve 1 g of 7-hydroxyethylcamptothecin in 50 mL of dry pyridine, add 2 g of succinic anhydride, stir and reflux for 24 hours, then add 2 g of succinic anhydride, and continue stirring and reflux for 24 hours. The reaction mixture was poured into a separatory funnel, 50 mL of distilled water was added, the aqueous solution was extracted with dichloromethane (200 mL×3), combined, dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure. It was separated by silica gel column chromatography and eluted with dichloromethane / methanol 50:1.5 to obtain 750 mg of light yellow solid (62% yield).
[0034] 1 H-NMR (400MHz, DMSO-d 6 , ppm): δ8.31 (1H, d, J = 8.4Hz), 8.22 (1H, d, J = 7.7Hz), 7.91 (1H, dd, J = 8.4, 7.0Hz), 7.78 (1H, dd, J=8.4, 7.0Hz), 7.35(1H, s), 5.40(2H, s), 5.33(2H, s), 4.40(2H, t), 3.53(2H, t), 3.38(2H, m), 3.36 (2H, m), 1.88 (2H, q, J=7.0 Hz), 0.89 (3H, t, J=7.0 Hz).
...
Embodiment 3
[0037] Preparation of 7-Hydroxyethylcamptothecin Succinate
[0038] Dissolve 1 g of 7-hydroxyethylcamptothecin in 40 mL of dry dimethyl sulfoxide, add 2 g of succinic anhydride, and then add 2 mL of dry pyridine, then stir and reflux for 12 hours. The reaction mixture was poured into a separatory funnel, 50 mL of distilled water was added, the aqueous solution was extracted with dichloromethane (200 mL×3), combined, dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure. It was separated by silica gel column chromatography and eluted with dichloromethane / methanol 50:1.5 to obtain 840 mg of a light yellow solid (66% yield).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com