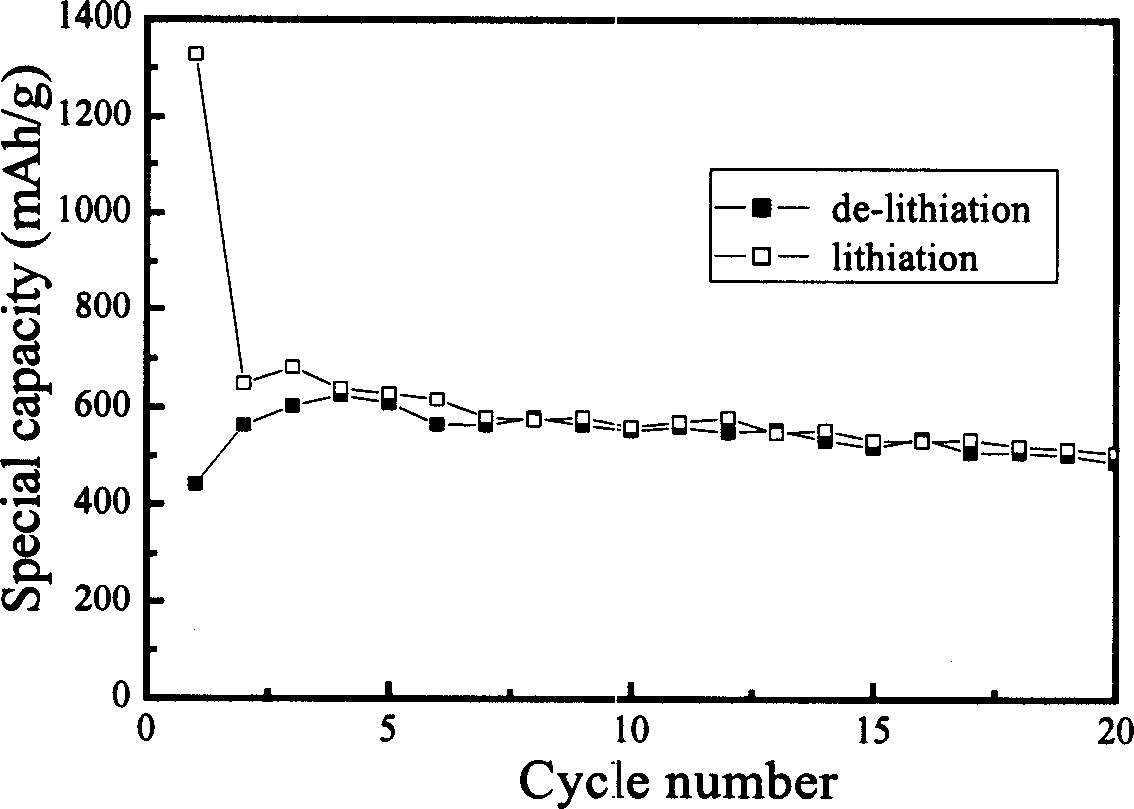
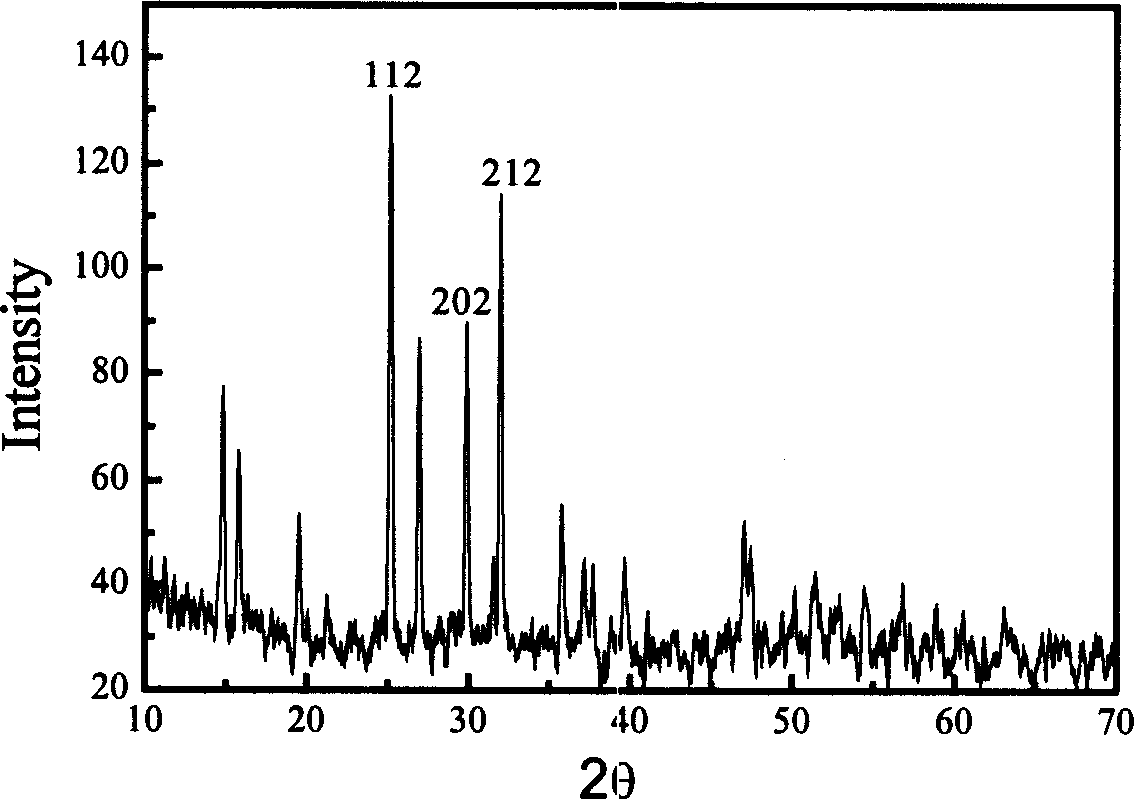
Preparing method for negative material of high-capacity tin-base lithium ion battery
A technology for ion batteries and negative electrode materials, applied in electrode manufacturing, battery electrodes, secondary batteries, etc., can solve the problems of relatively high equipment requirements, inability to completely eliminate volume effect, and high cost, so as to alleviate electrode volume changes and reduce irreversible The effect of capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation A solution: 0.07M SnCl 2 Solution 50ml, adding potassium sodium tartrate, the concentration is 0.2M, sucrose, the concentration is 0.01M; preparation B solution: 0.1M KBH 4 Solution 30ml, add ammonia water, ammonia concentration is 2M. Slowly add solution B to solution A dropwise while vigorously stirring solution A. After the dropwise addition was completed, the stirring was continued for 2 hours, during which the temperature was 25°C. The reaction solution was aged for 1 hour, and the resulting white precipitate was filtered, washed with deionized water until neutral, and dried at 90°C to obtain the product. Electrodes were prepared according to the method described above for electrochemical performance testing.
Embodiment 2
[0029] Preparation A solution: 0.07M SnCl 2Solution 50ml, add sodium citrate to it, the concentration is 0.2M, add ethylene glycol 2ml; preparation B solution: 0.1M KBH 4 Solution 30ml, which is added with urea, the concentration is 0.4M. Slowly add solution B to solution A dropwise while vigorously stirring solution A. After the dropwise addition was completed, the stirring was continued for 2 hours, during which the temperature was 65°C. The reaction solution was aged for 3 hours, and the resulting white precipitate was filtered, washed with deionized water until neutral, and dried at 90°C to obtain the product. Electrodes were prepared according to the method described above for electrochemical performance testing.
Embodiment 3
[0031] Formulation A: Solution 0.06M SnCl 2 Solution 50ml, add tartaric acid, the concentration is 0.2M, add ethylene glycol 1ml; preparation B solution: 0.07M KBH 4 Solution 30ml, wherein added ammonia water, ammonia concentration is 1M. Slowly add solution B to solution A dropwise while vigorously stirring solution A. After the dropwise addition was completed, the stirring was continued for 1.5 hours, during which the temperature was 45°C. The reaction solution was aged for 10 hours, and the resulting white precipitate was filtered, washed with deionized water until neutral, and dried at 90°C to obtain the product. Electrodes were prepared according to the method described above for electrochemical performance testing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com